Modern reaction-based indicator systems
- PMID: 19587959
- PMCID: PMC2862969
- DOI: 10.1039/b804436h
Modern reaction-based indicator systems
Abstract
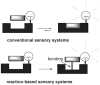
Traditional analyte-specific synthetic receptors or sensors have been developed on the basis of supramolecular interactions (e.g., hydrogen bonding, electrostatics, weak coordinative bonds). Unfortunately, this approach is often subject to limitations. As a result, increasing attention within the chemical sensor community is turning to the use of analyte-specific molecular indicators, wherein substrate-triggered reactions are used to signal the presence of a given analyte. This tutorial review highlights recent reaction-based indicator systems that have been used to detect selected anions, cations, reactive oxygen species, and neutral substrates.
Figures
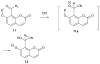
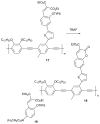
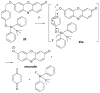
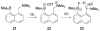
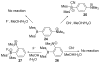
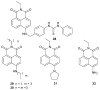


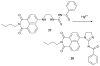
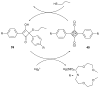
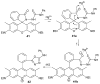
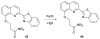
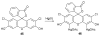
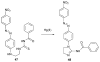
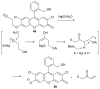
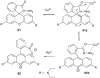
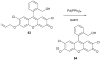
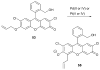
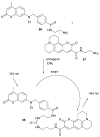

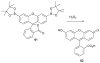
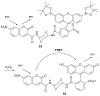
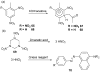

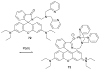
References
-
- Arigaand K, Kunitake T. Supramolecular Chemistry: Fundamentals and Applications. Springer; Heidelberg: 2006.
-
-
A considerable body of literature exists that deals with this topic. See, for instance: Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. The Royal Society of Chemistry; Cambridge, UK: 2006. Beer PD, Gale PA. Angew Chem, Int Ed. 2001;41:486–516.Valeur B, Leray I. Coord Chem Rev. 2000;205:3–40.
-
-
-
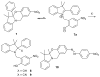
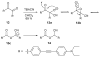
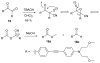
Cyanohydrin reaction-based indicators differ from most other systems considered in this review in that they are for the most part based on reactions that are reversible.
-
-
- Mohr GJ. Chem–Eur J. 2004;10:1082–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

