A mechanism of nucleocytoplasmic trafficking for the homeodomain protein PRH
- PMID: 19588232
- PMCID: PMC4440652
- DOI: 10.1007/s11010-009-0188-0
A mechanism of nucleocytoplasmic trafficking for the homeodomain protein PRH
Abstract
Proline-rich homeodomain (PRH)/hematopoietically expressed homeodomain (Hex) is a homeodomain protein that plays an important role in early embryonic patterning and hematopoiesis. PRH can act as either a tumor suppressor or an oncogene and its expression is dysregulated in certain types of lymphoid and myeloid leukemias. Aberrant exclusion of PRH from the nuclei has been associated with thyroid and breast cancers and a subset of myeloid leukemias. Accordingly, nuclear localization of PRH was found to be necessary for the inhibition of eIF4E-dependent transformation. Since PRH's nuclear-cytoplasmic localization has been associated with neoplastic transformation we sought to better understand how PRH is transported to the nuclear compartment. Here, we report an essential element that controls the mechanism of PRH nucleocytoplasmic trafficking, namely that it is imported into the nuclei by Karyopherin/Importin 7. Kap7 was identified as a binding partner for PRH in a GST-pull down from a HeLa cell protein lysate, followed by mass-spectrometry. The Kap7-PRH complex is dissociated in the presence of RanGTP, as expected for a nuclear import complex. Kap7 can bind directly to PRH in a GST-pull down assay with purified proteins, as well as mediates the transport of PRH to the nuclear compartment in a digitonin permeabilized cells assay. Finally, in vivo depletion of Kap7 dramatically reduces accumulation of PRH in the nucleus. Our data open the way for investigations of the mechanism of perturbed PRH localization in tumors and possible therapeutic interventions.
Figures



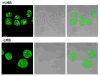
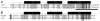
Similar articles
-
The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth.EMBO J. 2003 Feb 3;22(3):689-703. doi: 10.1093/emboj/cdg069. EMBO J. 2003. PMID: 12554669 Free PMC article.
-
Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2.EMBO J. 2000 Oct 16;19(20):5502-13. doi: 10.1093/emboj/19.20.5502. EMBO J. 2000. PMID: 11032817 Free PMC article.
-
Facilitated nucleocytoplasmic shuttling of the Ran binding protein RanBP1.Mol Cell Biol. 2000 May;20(10):3510-21. doi: 10.1128/MCB.20.10.3510-3521.2000. Mol Cell Biol. 2000. PMID: 10779340 Free PMC article.
-
Nucleocytoplasmic transport: Ran, beta and beyond.Trends Cell Biol. 2001 Dec;11(12):497-503. doi: 10.1016/s0962-8924(01)02144-4. Trends Cell Biol. 2001. PMID: 11719056 Review.
-
Nucleocytoplasmic transport enters the atomic age.Curr Opin Cell Biol. 2001 Jun;13(3):310-9. doi: 10.1016/s0955-0674(00)00213-1. Curr Opin Cell Biol. 2001. PMID: 11343901 Review.
Cited by
-
Nuclear localization of Obox4 is dependent on its homeobox domain.Clin Exp Reprod Med. 2013 Mar;40(1):1-6. doi: 10.5653/cerm.2013.40.1.1. Epub 2013 Mar 31. Clin Exp Reprod Med. 2013. PMID: 23614109 Free PMC article.
-
Biological functions and research progress of eIF4E.Front Oncol. 2023 Aug 3;13:1076855. doi: 10.3389/fonc.2023.1076855. eCollection 2023. Front Oncol. 2023. PMID: 37601696 Free PMC article. Review.
-
Karyopherins in nuclear transport of homeodomain proteins during development.Biochim Biophys Acta. 2011 Sep;1813(9):1654-62. doi: 10.1016/j.bbamcr.2011.01.013. Epub 2011 Jan 20. Biochim Biophys Acta. 2011. PMID: 21256166 Free PMC article. Review.
References
-
- Martinez Barbera JP, Clements M, Thomas P, et al. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. - PubMed
-
- Manfioletti G, Gattei V, Buratti E, et al. Differential expression of a novel proline-rich homeobox gene (Prh) in human hematolymphopoietic cells. Blood. 1995;85:1237–1245. - PubMed
-
- Jayaraman PS, Frampton J, Goodwin G. The homeodomain protein PRH influences the differentiation of haematopoietic cells. Leuk Res. 2000;24:1023–1031. - PubMed
-
- Hromas R, Radich J, Collins S. PCR cloning of an orphan homeobox gene (PRH) preferentially expressed in myeloid and liver cells. Biochem Biophys Res Commun. 1993;195:976–983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

