Mifepristone for induction of labour
- PMID: 19588336
- PMCID: PMC3992376
- DOI: 10.1002/14651858.CD002865.pub2
Mifepristone for induction of labour
Abstract
Background: The steroid hormone, progesterone, inhibits contractions of the pregnant uterus at all gestations. Antiprogestins (including mifepristone) have been developed to antagonise the action of progesterone, and have a recognised role in medical termination of early or mid-trimester pregnancy. Animal studies have suggested that mifepristone may also have a role in inducing labour in late pregnancy.
Objectives: To determine the effects of mifepristone for third trimester cervical ripening or induction of labour.
Search strategy: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register and reference lists of relevant papers (May 2009).
Selection criteria: Clinical trials comparing mifepristone used for third trimester cervical ripening or labour induction with placebo/no treatment or other labour induction methods.
Data collection and analysis: A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two-stage method of data extraction. For this update, two review authors independently assessed trial quality and extracted data.
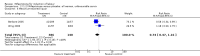
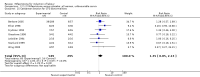
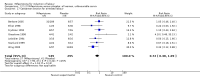
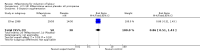
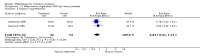
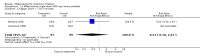
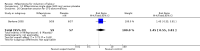
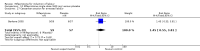
Main results: Ten trials (1108 women) are included. Compared to placebo, mifepristone treated women were more likely to be in labour or to have a favourable cervix at 48 hours (risk ratio (RR) 2.41, 95% confidence intervals (CI) 1.70 to 3.42) and this effect persisted at 96 hours (RR 3.40, 95% CI 1.96 to 5.92). They were less likely to need augmentation with oxytocin (RR 0.80, 95% CI 0.66 to 0.97). Mifepristone treated women were less likely to undergo caesarean section (RR 0.74, 95% CI 0.60 to 0.92) but more likely to have an instrumental delivery (RR 1.43, 95% CI 1.04 to 1.96). Women receiving mifepristone were less likely to undergo a caesarean section as a result of failure to induce labour (RR 0.40, 95% CI 0.20 to 0.80). There is insufficient evidence to support a particular dose but a single dose of 200 mg mifepristone appears to be the lowest effective dose for cervical ripening (increased likelihood of cervical ripening at 72 hours (RR 2.13, 95% CI 1.15 to 3.97). Abnormal fetal heart rate patterns were more common after mifepristone treatment (RR 1.85, 95% CI 1.17 to 2.93), but there was no evidence of differences in other neonatal outcomes. There is insufficient information on the occurrence of uterine rupture/dehiscence in the reviewed studies.
Authors' conclusions: There is insufficient information available from clinical trials to support the use of mifepristone to induce labour. However, the studies suggest that mifepristone is better than placebo in reducing the likelihood of caesarean sections being performed for failed induction of labour; therefore, this may justify future trials comparing mifepristone with the routine cervical ripening agents currently in use. There is little information on effects on the baby.
Conflict of interest statement
None known.
Figures








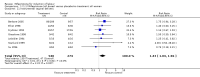













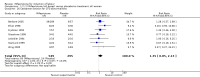
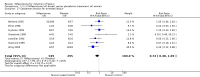







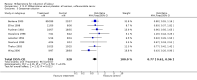
























































































































Update of
-
Mifepristone for induction of labour.Cochrane Database Syst Rev. 2000;(4):CD002865. doi: 10.1002/14651858.CD002865. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2009 Jul 08;(3):CD002865. doi: 10.1002/14651858.CD002865.pub2. PMID: 11034779 Updated.
Similar articles
-
Mifepristone for induction of labour.Cochrane Database Syst Rev. 2000;(4):CD002865. doi: 10.1002/14651858.CD002865. Cochrane Database Syst Rev. 2000. Update in: Cochrane Database Syst Rev. 2009 Jul 08;(3):CD002865. doi: 10.1002/14651858.CD002865.pub2. PMID: 11034779 Updated.
-
Acupuncture or acupressure for induction of labour.Cochrane Database Syst Rev. 2017 Oct 17;10(10):CD002962. doi: 10.1002/14651858.CD002962.pub4. Cochrane Database Syst Rev. 2017. PMID: 29036756 Free PMC article.
-
Methods of term labour induction for women with a previous caesarean section.Cochrane Database Syst Rev. 2017 Jun 9;6(6):CD009792. doi: 10.1002/14651858.CD009792.pub3. Cochrane Database Syst Rev. 2017. PMID: 28599068 Free PMC article.
-
Pharmacological and mechanical interventions for labour induction in outpatient settings.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD007701. doi: 10.1002/14651858.CD007701.pub3. Cochrane Database Syst Rev. 2017. PMID: 28901007 Free PMC article.
-
Mechanical methods for induction of labour.Cochrane Database Syst Rev. 2001;(4):CD001233. doi: 10.1002/14651858.CD001233. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2012 Mar 14;(3):CD001233. doi: 10.1002/14651858.CD001233.pub2. PMID: 11687101 Updated.
Cited by
-
Cervix Stromal Cells and the Progesterone Receptor A Isoform Mediate Effects of Progesterone for Prepartum Remodeling.Reprod Sci. 2019 May;26(5):690-696. doi: 10.1177/1933719118820462. Epub 2019 Jan 17. Reprod Sci. 2019. PMID: 30654718 Free PMC article.
-
Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts.Pharmaceuticals (Basel). 2023 Jul 8;16(7):982. doi: 10.3390/ph16070982. Pharmaceuticals (Basel). 2023. PMID: 37513894 Free PMC article.
-
Progestin therapy to prevent preterm birth: History and effectiveness of current strategies and development of novel approaches.Placenta. 2019 Apr;79:46-52. doi: 10.1016/j.placenta.2019.01.018. Epub 2019 Jan 28. Placenta. 2019. PMID: 30745115 Free PMC article. Review.
-
Comparison of outpatient with inpatient mifepristone usage for cervical ripening: A randomised controlled trial.Eur J Obstet Gynecol Reprod Biol X. 2023 May 16;18:100198. doi: 10.1016/j.eurox.2023.100198. eCollection 2023 Jun. Eur J Obstet Gynecol Reprod Biol X. 2023. PMID: 37234794 Free PMC article.
-
Clinical Utility of Mifepristone: Apprising the Expanding Horizons.Cureus. 2022 Aug 23;14(8):e28318. doi: 10.7759/cureus.28318. eCollection 2022 Aug. Cureus. 2022. PMID: 36158399 Free PMC article. Review.
References
References to studies included in this review
Berkane 2005 {published data only}
-
- Berkane N, Verstraete L, Uzan S, Boog G, Maria B. Use of mifepristone to ripen the cervix and induce labor in term pregnancies. American Journal of Obstetrics and Gynecology 2005;192(1):114‐20. - PubMed
Elliot 1998 {published data only}
-
- Elliot C, Brennand JE, Calder AA. The effect of mifepristone (RU486) on cervical ripening and induction of labour in human pregnancy at term. 27th British Congress of Obstetrics & Gynaecology; 1995; 4‐7 July, Dublin. 1995:207.
-
- Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstetrics & Gynecology 1998;92(5):804‐9. - PubMed
Frydman 1992 {published data only}
-
- Frydman R, Baton C, Lelaidier C, Vial M, Bourget P, Fernandez H. Mifepristone for induction of labour. Lancet 1991;337:488‐9. - PubMed
-
- Frydman R, Lelaidier C, Baton‐Saint‐Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double blind, randomized, placebo‐controlled study. Obstetrics & Gynecology 1992;80:972‐5. - PubMed
-
- Frydman R, Lelaidier C, Baton‐Saint‐Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double‐blind, randomized, placebo‐controlled study. International Journal of Gynaecology & Obstetrics 1993;42:220. - PubMed
-
- Frydman R, Taylor S, Paoli C, Pourade A. Ru 486 (mifepristone): A new tool for labour induction in term women with fetus alive [Le RU 486 (mifepristone) un novel outil pour le declenchment du travail a terme]. Contraception, Fertilite, Sexualite 1992;20(12):1133‐6. - PubMed
-
- Lelaidier C, Benifla JL, Fernandez H, Baton C, Borget P, Bourrier MC, et al. RU 486 (mifepristone) in medical indications for labour induction in pregnancies at term: results of a randomized, double‐blind study of RU 486 vs placebo [Interet du RU 486 (Mifepristone) dans les indications medicales de declenchement du travail a terme]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1993;22:91‐100. - PubMed
Giacalone 1998 {published data only}
-
- Giacalone PL, Targosz V, Laffargue F, Boog G, Faure JM. Cervical ripening with mifepristone before labor induction: a randomized study. Obstetrics & Gynecology 1998;92(4 Pt 1):487‐92. - PubMed
Lelaidier 1994 {published data only}
-
- Lelaidier C, Baton C, Benifla JL, Fernandez H, Bourget P, Frydman R. Mifepristone for labour induction after previous caesarean section. British Journal of Obstetrics and Gynaecology 1994;101:501‐3. - PubMed
Stenlund 1999 {published data only}
-
- Stenlund PM, Bygdeman M, Ekman G. Induction of labor with mifepristone (RU 486). A randomized double‐blind study in post‐term pregnant women with unripe cervices. Acta Obstetricia et Gynecologica Scandinavica. Supplement 1994;73(161):Abstract no: FP50. - PubMed
-
- Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone ‐ a randomized, double‐blind study versus placebo. Acta Obstetricia et Gynecologica Scandinavica 1999;78:793‐8. - PubMed
Su 1996 {published data only}
-
- Su H, Li E, Weng L. Mifepristone for induction of labor. Chinese Journal of Obstetrics and Gynaecology 1996;31:676‐80. - PubMed
Thakur 2005 {published data only (unpublished sought but not used)}
-
- Thakur V, Dorman E, Sanu L, Harrington K. Mifepristone is an effective ripening agent in postdates primips with cervical length >2.5cm, but mode of delivery correlates with birthweight: a randomised, placebo controlled double blind study. Ultrasound in Obstetrics and Gynecology 2005;26:452.
Wing 2000 {published data only}
-
- Byrne JD, Wing DA, Fraser M, Fassett MJ, Goodwin TM, Challis JRG. Mifepristone: effect on plasma corticotropin‐releasing hormone, adrenocorticotropin hormone, and cortisol in term pregnancy. Journal of Perinatology 2004;24(7):416‐20. - PubMed
-
- Fassett MJ, Lachelin GC, McGarrigle HH, Wing DA. Alterations in saliva steroid hormone levels after oral mifepristone administration in women with pregnancies of greater than 41 weeks' gestation. Reproductive Sciences 2008;15(4):394‐9. - PubMed
-
- Fassett MJ, Wing DA. Salivary estriol/progesterone ratio and the success of labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S210.
-
- Fassett MJ, Wing DA. Uterine activity after oral mifepristone administration in human pregnancies beyond 41 weeks' gestation. Gynecologic and Obstetric Investigation 2008; Vol. 65, issue 2:112‐5. - PubMed
-
- Wing D, Fassett M, Mishell DR. Mifepristone for preinduction cervical ripening beyond 41 weeks gestation: A randomised controlled trial. Obstetrics & Gynecology 2000;96(4):543‐48. - PubMed
Wing 2005 {published data only}
-
- Wing D, Guberman C, Fassett M. A comparison of oral mifepristone to intravenous oxytocin for pre‐induction cervical ripening and labour induction in women with pre‐labour rupture of membranes beyond 36 weeks gestation. American Journal of Obstetrics & Gynecology 2003;189(6 Suppl 1):S204. - PubMed
-
- Wing DA, Guberman C, Fassett M. A randomized comparison of oral mifepristone to intravenous oxytocin for labor induction in women with prelabor rupture of membranes beyond 36 weeks' gestation. American Journal of Obstetricians & Gynaecologists 2005;192:445‐51. - PubMed
References to studies excluded from this review
Cabrol 1990 {published data only}
-
- Cabrol D, Dubois C, Cronje H Gonnet JM, Guillot M, Maria, B, et al. Induction of labor with mifepristone (RU 486) in intrauterine fetal death. American Journal of Obstetrics and Gynecology 1990;163:540‐2. - PubMed
Jiang 1997 {published data only}
-
- Jiang X, Wang H, Zhang Z. Determination of fetal umbilical artery flow velocity during induction of term labour by mifepristone. Chinese Journal of Obstetrics & Gynecology 1997;32(12):732‐4. - PubMed
Li 1996 {published data only}
-
- Li L, Gao W, Chen S. Labour induction in women at term with mifepristone and misoprostol. Chung Hua Fu Chan Ko Tsa Chih 1996;31:681‐4. - PubMed
Padayachi 1988 {published data only}
-
- Padayachi T, Norman RJ, Moodley J, Heyns A. Mifepristone and induction of labour in second half of pregnancy. Lancet 1988;1:647. - PubMed
Additional references
Alfirevic 2006
Bartley 2000
-
- Bartley J, Tong S, Everington D, Baird DT. Parity is a major determinant of success rate in medical abortion: a retrospective analysis of 3161 consecutive cases of early medical abortion treated with reduced doses of mifepristone and vaginal gemeprost. Contraception 2000;62(6):297‐303. - PubMed
Boulvain 2001
Boulvain 2005
Boulvain 2008
Bricker 2000
Clarke 1999
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.0 [updated July 1999]. In: Review Manager (RevMan) [Computer program]. Version 4.0 Oxford, England: The Cochrane Collaboration, 1999.
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. - PubMed
Dlamini 1995
-
- Dlamini BJ, Anderson LL. Mifepristone (RU 486) induces parturition in primiparous beef heifers and reduces incidence of dystocia. Journal of Animal Science 1995;73:3421‐6. - PubMed
Dudley 1996
-
- Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biology of Reproduction 1996;55:992‐5. - PubMed
Fairley 2005
-
- Fairley TE, MacKenzie M, Owen P, MacKenzie F. Management of late intrauterine death using a combination of mifepristone and misoprostol‐‐experience of two regimens. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;118(1):28‐31. - PubMed
Fang 1997
-
- Fang X, Wong S, Mitchell BF. Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology 1997;138:2763‐8. - PubMed
French 2001
Haluska 1994
-
- Hluska GJ, Kaler CA, Cook MJ, Novy MJ. Prostaglandin production during spontaneous labor and after treatment with RU486 in pregnant rhesus macaques. Biology of Reproduction 1994;51:760‐5. - PubMed
Hapangama 2003
-
- Hapangama DK. Mifepristone: the multifaceted antihormone. Journal of Drug Evaluation 2003;1(5):149‐75.
Heikinheimo 1997
-
- Heikinheimo O. Clinical pharmacokinetics of mifepristone. Clinical Pharmacokinetics 1997;33:7‐17. - PubMed
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008].The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hofmeyr 2003a
Hofmeyr 2003b
-
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002074] - DOI
Howarth 2001
Hutton 2001
Kavanagh 2001
Kavanagh 2005
Kavanagh 2006a
Kavanagh 2006b
Kelly 2001a
Kelly 2001b
Kelly 2001c
Kelly 2003
Kelly 2008a
Luckas 2000
Muzonzini 2004
Neilson 2000
RevMan 2008 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Smith 2003
Smith 2004
Thomas 2001
Van Look 1995
-
- Look PF, Hertzen H. Clinical uses of antiprogestogens. Human Reproduction Update 1995;1:19‐34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

