Single dose oral etodolac for acute postoperative pain in adults
- PMID: 19588426
- PMCID: PMC4164827
- DOI: 10.1002/14651858.CD007357.pub2
Single dose oral etodolac for acute postoperative pain in adults
Abstract
Background: Etodolac is a selective cyclo-oxygenase-2 (COX-2) inhibitor, with evidence of efficacy in osteoarthritis and rheumatoid arthritis. Its analgesic efficacy in postoperative pain has not been clearly established. There are no systematic reviews on Etodolac's use in this condition.
Objectives: To assess the analgesic efficacy of etodolac in single oral doses for moderate and severe postoperative pain.
Search strategy: We searched Cochrane CENTRAL, MEDLINE, EMBASE and the Oxford Pain Relief Database for studies to May 2009.
Selection criteria: Randomised, double blind, placebo-controlled trials of single dose orally administered etodolac (any formulation) in adults with moderate to severe acute postoperative pain.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. Pain relief or pain intensity data were extracted and converted into the dichotomous outcome of number of participants with at least 50% pain relief over 4 to 6 hours, from which relative risk (RR) and number needed to treat to benefit (NNT) were calculated. Numbers of participants using rescue medication over specified time periods, and time to use of rescue medication, were sought as additional measures of efficacy. Information on adverse events and withdrawals were collected.
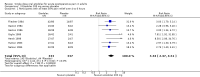
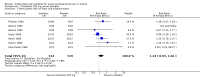
Main results: Nine studies (1459 participants) compared etodolac and placebo. Studies were of adequate reporting quality, and the majority of participants had pain following dental extractions. The dose of etodolac used was 25 mg to 1200 mg, with most of the information for 100 mg and 200 mg. For at least 50% pain relief over 4 to 6 hours compared with placebo the NNT for etodolac 100 mg (498 participants) was 4.8 (3.5 to 7.8) and for etodolac 200 mg (670 participants) it was 3.3 (2.7 to 4.2). Very limited information with the extended release formulation did not suggest improved benefit for this outcome.The proportion of participants with at least 50% pain relief was 41% with 100 mg and 44% with 200 mg. Remedication was needed by about 60% with etodolac 200 mg or 400 mg over 6 to 8 hours, compared with almost 80% with placebo.Adverse events were uncommon, and not significantly different form placebo.
Authors' conclusions: Etodolac 200 mg may be a useful analgesic in postoperative pain, with efficacy similar to paracetamol 1000 mg and celecoxib 200 mg. Higher doses may provide analgesia equivalent to more commonly used drugs, such as ibuprofen 400 mg, naproxen 500 mg and diclofenac 50 mg.
Conflict of interest statement
RAM, HJM and SD have received research support from charities, government and industry sources at various times, but no such support was received for this work. RAM and HJM have consulted for various pharmaceutical companies. RAM, and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions.
Figures



















Update of
- doi: 10.1002/14651858.CD007357
References
References to studies included in this review
Fliedner 1984 {published data only}
-
- Fliedner L, Levsky M, Kechejian H. Analgesia with etodolac in oral postsurgical pain. Current Therapeutic Research 1984;36(1):33‐45.
Friedrich 1983 {published data only}
-
- Friedrich E. A comparison of etodolac (Ultradol) with aspirin and placebo in patients with episiotomy pain. Current Therapeutic Research ‐ Clinical and Experimental 1983;33(1):100‐7.
Gaston 1984 {published data only}
-
- Gaston GW, Mallow RD, Frank JE. The efficacy of etodolac for patients with pain following oral surgery. Journal of Oral and Maxillofacial Surgery 1984;42(6):362‐6. - PubMed
Gaston 1986 {published data only}
-
- Gaston GW, Mallow RD, Frank JE. Comparison of etodolac, aspirin and placebo for pain after oral surgery. Pharmacotherapy 1986;6(5):199‐205. - PubMed
Giglio 1986 {published data only}
-
- Giglio JA, Campbell RL. Comparison of etodolac, zomepirac, and placebo for relief of pain after oral surgery. Journal of Oral & Maxillofacial Surgery 1986;44(10):765‐70. - PubMed
Hersh 1999 {published data only}
-
- Hersh EV, Levin LM, Cooper SA, Reynolds D, Gallegos LT, McGoldrick K, et al. Conventional and extended‐release etodolac for postsurgical dental pain. Clinical therapeutics 1999;21(8):1333‐42. [PUBMED: 10485505] - PubMed
Hutton 1983 {published data only}
-
- Hutton CE. The effectiveness of 100 and 200 mg etodolac (Ultradol), aspirin, and placebo in patients with pain following oral surgery. Oral Surgery, Oral Medicine, and Oral Pathology 1983;56(6):575‐80. - PubMed
Nelson 1985 {published data only}
Versichelen 1982 {published data only}
-
- Versichelen L, Bilsback P, Rolly G, Merlo M, Joubert L. Etodolac in postsurgical pain: a double‐blind dose‐ranging efficacy study with aspirin and placebo. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1982;20(5):236‐9. [PUBMED: 6212554] - PubMed
References to studies excluded from this review
Apaydin 1994 {published data only}
-
- Apaydin A, Ozyuvaci H, Ordulu M, Disci R. Postoperative pain relief by single dose Diclofenac Kalium and Etodolac. A comparative clinical study. Agri Dergisi: The Journal of The Turkish Society of Algology 1994;6(4):28‐34.
Boni 1999 {published data only}
-
- Boni J, Korth‐Bradley J, McGoldrick K, Appel A, Cooper S. Pharmacokinetic and pharmacodynamic action of etodolac in patients after oral surgery. Journal of Clinical Pharmacology 1999;39(7):729‐37. [PUBMED: 10392328] - PubMed
Koizuka 2004 {published data only}
-
- Koizuka S, Saito S, Obata H, Sasaki M, Nishikawa K, Takahashi K, et al. Oral etodolac, a COX‐2 inhibitor, reduces postoperative pain immediately after fast‐track cardiac surgery. Journal of Anesthesia 2004;18(1):9‐13. [PUBMED: 14991469] - PubMed
Lin 2006 {published data only}
-
- Lin S, Levin L, Emodi O, Abu El‐Naaj I, Peled M. Etodolac versus dexamethasone effect in reduction of postoperative symptoms following surgical endodontic treatment: a double‐blind study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2006;101(6):814‐7. [PUBMED: 16731406] - PubMed
Mizraji 1990 {published data only}
-
- Mizraji M. Clinical response to etodolac in the management of pain. European Journal of Rheumatology and Inflammation 1990;10(1):35‐43. [PUBMED: 1699764] - PubMed
Scott 1986 {published data only}
-
- Scott R, Ellis E 3rd, Upton LG. Double‐blind evaluation of etodolac (200 mg, 400 mg) compared with zomepirac (100 mg) and placebo on third molar extraction pain. Oral Surgery, Oral Medicine, Oral Pathology 1986;62(6):638‐42. - PubMed
Additional references
Chen 2008
-
- Chen Y‐F, Jobanputra P, Barton P, Bryan S, Fry‐Smith A, Harris G, et al. Cyclooxygenase‐2 selective non‐steroidal anti‐inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technology Assessment 2008; Vol. 12:11. - PubMed
Clarke 2009
Collins 1997
-
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. - PubMed
Collins 2001
-
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91:189‐94. - PubMed
Cook 1995
Cooper 1991
-
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The Design of Analgesic Clinical Trials (Advances in Pain Research and Therapy Vol. 18). New York: Raven Press, 1991:117‐24.
Derry 2008
Derry C 2009a
Derry C 2009b
Derry P 2009
Edwards 1999
-
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427‐37. - PubMed
Forrest 2002
-
- Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. British Journal of Anaesthesia 2002;88(2):227‐33. - PubMed
Garcia Rodriguez 2008
-
- García Rodríguez LA, Tacconelli S, Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti‐inflammatory drugs in the general population. Journal of the American College of Cardiology 2008;52(20):1628‐36. [DOI: 10.1016/j.jacc.2008.08.041] - DOI - PubMed
Garner 2002
Grahame‐Smith 2002
-
- Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy. 3. Oxford: Oxford University Press, 2002. [ISBN: 13: 978‐0‐19‐263234‐0]
Hawkey 1999
-
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. - PubMed
Hawkey 2001
-
- Hawkey CJ, Jones JI. Gastrointestinal safety of COX‐2 specific inhibitors. Gastroenterology Clinics of North America 2001;30(4):921‐36. - PubMed
Hawkey 2006
-
- Hawkey CJ. NSAIDs, coxibs, and the intestine. Journal of Cardiovascular Pharmacology 2006;47(Suppl 1):S72‐5. - PubMed
Jadad 1996a
-
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kearney 2006
-
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo‐oxygenase‐2 inhibitors and traditional non‐steroidal anti‐inflammatory drugs increase the risk of atherothrombosis? Meta‐analysis of randomised trials. BMJ (Clinical research ed.) 2006;3(332):1302‐8. [DOI: 10.1136/bmj.332.7553.1302] - DOI - PMC - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. - PubMed
Lloyd 2009
McQuay 1982
McQuay 2005
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of meta‐analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354:1896‐900. - PubMed
Moore 1996
Moore 1997a
Moore 1997b
Moore 1998
Moore 2003
-
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7]
Moore 2005
Moore 2005b
-
- Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta‐analysis of information from company clinical trial reports. Arthritis Research & Therapy 2005;7(3):R644‐65. [DOI: 10.1186/ar1704] - DOI - PMC - PubMed
Moore 2006
-
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2]
Morris 1995
PACT 2007
-
- Anonymous. Prescription cost analysis, England 2007. NHS Information Centre, 2007. [ISBN:978‐1‐84636‐210‐1]
Straube 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

