Selective upregulation of interleukin-8 by human rhabdomyosarcomas in response to hypoxia: therapeutic implications
- PMID: 19588509
- PMCID: PMC4021846
- DOI: 10.1002/ijc.24732
Selective upregulation of interleukin-8 by human rhabdomyosarcomas in response to hypoxia: therapeutic implications
Abstract
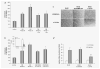
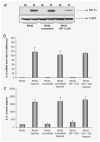
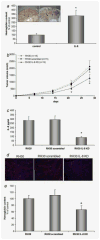
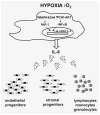
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma of adolescence and childhood. Because RMS tumors are highly vascularized, we sought to determine which factors secreted by RMS cells are crucial in stimulating angiogenesis in response to hypoxia. To address this issue, we evaluated expression of several proangiogenic factors [interleukin (IL)-8, vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)-2, stromal-derived factor (SDF)-1, hepatocyte growth factor (HGF) and leukemia inhibitory factor (LIF)] in 8 human RMS cell lines in both normal steady-state and hypoxic conditions. We found by real-time quantitative polymerase chain reaction (RQ-PCR) and confirmed by enzyme-linked immunosorbent assay (ELISA) that from all the factors evaluated, IL-8, whose expression is very low in normoxia, had been very highly expressed and secreted by RMS cells lines during hypoxic conditions ( approximately 40-170 times). Interestingly, this upregulation was not affected by knocking down hypoxia-inducible factor (HIF)-1alpha, but was inhibited by mitogen-activated protein kinase (MAPK)p42/44 and phosphatidylinositaol 3-kinase (PI3K)/AKT pathway inhibitors. This suggests that IL-8 expression is regulated in an activating protein (AP)-1- and nuclear factor (NF)-kappaB-dependent manner. Furthermore, we found that conditioned media (CM) harvested from RMS cells exposed to hypoxia activated and stimulated chemotactic responses in human umbilical vein endothelial cells (HUVECs) and that IL-8 was responsible for hypoxia-related effects. Finally, by employing shRNA, the expression of IL-8 in human RH-30 cells was downregulated. We noticed that such RMS cells, if injected into skeletal muscles of immunodeficient mice, have a reduced ability for tumor formation. We conclude that IL-8 is a pivotal proangiogenic factor released by human RMS cells in hypoxic conditions and that the targeting of IL-8 may prove to be a novel and efficient strategy for inhibiting RMS growth.
Figures


Similar articles
-
Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy.Cancer Res. 2003 Nov 15;63(22):7926-35. Cancer Res. 2003. PMID: 14633723
-
RasGRF1 regulates proliferation and metastatic behavior of human alveolar rhabdomyosarcomas.Int J Oncol. 2012 Sep;41(3):995-1004. doi: 10.3892/ijo.2012.1536. Epub 2012 Jun 28. Int J Oncol. 2012. PMID: 22752028 Free PMC article.
-
VEGF production by primary human renal proximal tubular cells: requirement of HIF-1, PI3-kinase and MAPKK-1 signaling.Cell Physiol Biochem. 2005;15(1-4):99-108. doi: 10.1159/000083642. Cell Physiol Biochem. 2005. PMID: 15665520
-
Gambogic acid suppresses hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial growth factor expression via inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target protein of rapamycin pathway in multiple myeloma cells.Cancer Sci. 2014 Aug;105(8):1063-70. doi: 10.1111/cas.12458. Epub 2014 Jul 27. Cancer Sci. 2014. PMID: 24890366 Free PMC article.
-
Differential regulation of vascular endothelial growth factor by Akt and mammalian target of rapamycin inhibitors in cell lines derived from childhood solid tumors.Mol Cancer Ther. 2007 May;6(5):1620-8. doi: 10.1158/1535-7163.MCT-06-0646. Epub 2007 May 4. Mol Cancer Ther. 2007. PMID: 17483438
Cited by
-
The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature.Int J Mol Sci. 2021 Jan 15;22(2):843. doi: 10.3390/ijms22020843. Int J Mol Sci. 2021. PMID: 33467722 Free PMC article. Review.
-
Does it make sense to target one tumor cell chemotactic factor or its receptor when several chemotactic axes are involved in metastasis of the same cancer?Clin Transl Med. 2016 Dec;5(1):28. doi: 10.1186/s40169-016-0113-6. Epub 2016 Aug 10. Clin Transl Med. 2016. PMID: 27510263 Free PMC article. Review.
-
Tumor and Peripheral Immune Status in Soft Tissue Sarcoma: Implications for Immunotherapy.Cancers (Basel). 2021 Aug 1;13(15):3885. doi: 10.3390/cancers13153885. Cancers (Basel). 2021. PMID: 34359785 Free PMC article. Review.
-
Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1.Hum Mol Genet. 2018 Aug 15;27(16):2789-2804. doi: 10.1093/hmg/ddy192. Hum Mol Genet. 2018. PMID: 29771332 Free PMC article.
-
Thrombin regulates the metastatic potential of human rhabdomyosarcoma cells: distinct role of PAR1 and PAR3 signaling.Mol Cancer Res. 2010 May;8(5):677-90. doi: 10.1158/1541-7786.MCR-10-0019. Epub 2010 May 4. Mol Cancer Res. 2010. PMID: 20442298 Free PMC article.
References
-
- Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nature Genet. 1993;3:113–117. - PubMed
-
- Bennicelli JL, Advani S, Schafer BW, Barr FG. PAX3 and PAX7 exhibit conserved cis-acting transcription repression domains and utilize a common gain of function mechanism in alveolar rhabdomyosarcoma. Oncogene. 1999;18:4348–4356. - PubMed
-
- Collins MH, Zhao H, Womer RB, Barr FG. Proliferative and apoptotic differences between alveolar rhabdomyosarcoma subtypes: a comparative study of tumors containing PAX3-FKHR or PAX7-FKHR gene fusions. Med Ped Oncol. 2001;37:83–89. - PubMed
-
- Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994;54:2869–2872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

