Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group
- PMID: 19588519
- PMCID: PMC4408767
- DOI: 10.1002/pbc.22088
Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children's Oncology Group
Abstract
Background: PRETEXT is used to stratify risk in children with hepatoblastoma by the Liver Tumor Strategy Group (SIOPEL) of the International Society of Pediatric Oncology (SIOP). A recent analysis excluding patients that did not survive neoadjuvant chemotherapy, concluded that PRETEXT was superior to Children's Oncology Group (COG) stage for predicting survival. Puzzled by this result, we made a similar comparison of PRETEXT and COG stage. This time, however, we include all patients, and we compare predictive value at diagnosis, instead of after neoadjuvant chemotherapy.
Methods: Hepatoblastoma patients in INT-0098 were retrospectively reviewed for PRETEXT and other potential prognostic factors including pathologic subtype, and alpha-fetoprotein (AFP).
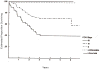
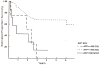
Results: Five-year overall survival by PRETEXT was 88.9%, 84.5%, 71.6%, and 30.9%, for PRETEXT I, II, III, and IV, respectively. The 5-year overall survival rates by COG stage were 100%, 97.5%, 100%, 70.2%, and 39.3% for Stage I pure fetal histology (PFH), Stage I unfavorable histology (UH = not PFH), Stage II, Stage III, and Stage IV, respectively. PRETEXT added significant additional prognostic information within the COG Stage III, but not COG Stage IV. Additional prognostic factors statistically significant for an increased risk of death were small-cell-undifferentiated (SCU) histologic subtype and AFP < 100 at diagnosis.
Conclusions: PRETEXT, COG stage, SCU histology, and AFP < 100, as assessed at diagnosis, are important determinants of survival that will allow us to better develop common international criteria for risk stratification. Common risk stratification is an essential prerequisite to establish effective cooperation across the ocean in this field of rare tumors.
Figures




References
-
- Evans AE, Land VJ, Newton WA, et al. Combination chemotherapy (vincristine, Adriamycin, Cyclophosphamide, and 5-fluorouracil) in the treatment of children with malignant hepatoma. Cancer. 1982;50:821–826. - PubMed
-
- Sasaki F, Matsunaga T, Iwafuchi M, et al. Outcome of hepatoblastoma treated with JPLT-1 (Japanese Study Group for Pediatric Liver Tumor) Protocol-1: A report from the Japanese Study Group for Pediatric Liver Tumor. J Pediatr Surg. 2002;37:851–856. - PubMed
-
- Ortega JA, Douglass EC, Feusner JH, et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665–75. - PubMed
-
- Reynolds M, Douglass EC, Finegold M, et al. Chemotherapy can convert unresectable hepatoblastoma. J Pediatr Surg. 1992;27:1080–1084. - PubMed
-
- Von Schweinitz D, Hecker H, Schmidt-von-Arndt G, et al. Prognostic factors and staging systems in childhood hepatoblastoma. Int J Cancer. 1997;74:593–599. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

