Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4
- PMID: 19589169
- PMCID: PMC2717941
- DOI: 10.1186/1743-422X-6-100
Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4
Abstract
Background: Proventricular dilatation disease (PDD) is a fatal disorder of psittacine birds worldwide. The disease is characterized by lymphoplasmacytic infiltration of the central and peripheral nervous systems, leading to gastrointestinal motility and/or central nervous system dysfunction. Recently, we detected a significant association between avian bornavirus (ABV) infection and clinical signs of PDD in psittacines. However, it remains unclear whether ABV infection actually causes PDD. To address this question, we examined the impact of ABV inoculation on the cockatiel (Nymphicus hollandicus).
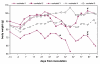
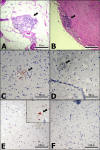
Results: Five cockatiels were inoculated via multiple routes (intramuscular, intraocular, intranasal, and oral) with a brain homogenate derived from either a PDD(+) avian bornavirus 4 (ABV4) (+) case (n = 3 inoculees) or from a PDD(-) ABV(-) control (n = 2 inoculees). The control birds remained free of clinical or pathological signs of PDD, and tested ABV(-) by RT-PCR and immunohistochemistry (IHC). In contrast, all three cockatiels inoculated with ABV4(+) brain homogenate developed gross and microscopic PDD lesions, and two exhibited overt clinical signs. In numerous tissues, ABV RT-PCR and sequence analysis demonstrated the presence of ABV4 RNA nearly identical to that in the inoculum. ABV was detected in the central nervous system of the three ABV-inoculees by IHC. Pyrosequencing to investigate the viral flora in the ABV4(+) inoculum uncovered 7 unique reads sharing 73-100% nucleotide sequence identity with previously identified ABV sequences and 24 reads sharing 40-89% amino acid sequence identity with viruses in the Retroviridae and Astroviridae families. Of these candidate viral species, only ABV RNA was recovered from tissues of the inoculated birds.
Conclusion: In this study, the clinical and pathological manifestations of PDD were induced by inoculation of cockatiels with brain homogenates containing avian bornavirus 4. By using high throughput pyrosequencing an in-depth view of the viral content of the inoculum was achieved, revealing that of 3 candidate virus families detected, only the presence of ABV RNA correlated with the development of PDD. This study provides evidence of a causal association between ABV4 infection and PDD in cockatiels.
Figures



References
-
- Daoust PY, Julian RJ, Yason CV, Artsob H. Proventricular impaction associated with nonsuppurative encephalomyelitis and ganglioneuritis in two Canada geese. J Wildl Dis. 1991;27:513–517. - PubMed
-
- Gregory C, Latimer KS, Niagro F, Ritchie BW, Campagnoli RP, Norton TM, Greenacre CB. A review of proventricular dilation syndrome. J Assoc Avian Vet. 1994;8:69–75.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

