Long range communication between exosites 1 and 2 modulates thrombin function
- PMID: 19589779
- PMCID: PMC2757964
- DOI: 10.1074/jbc.M109.000042
Long range communication between exosites 1 and 2 modulates thrombin function
Erratum in
-
Long range communication between exosites 1 and 2 modulates thrombin function.J Biol Chem. 2015 Feb 20;290(8):4814. doi: 10.1074/jbc.A109.000042. J Biol Chem. 2015. PMID: 25713406 Free PMC article. No abstract available.
Abstract
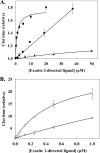
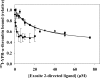
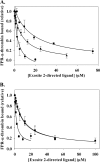
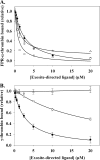
Although exosites 1 and 2 regulate thrombin activity by binding substrates and cofactors and by allosterically modulating the active site, it is unclear whether there is direct allosteric linkage between the two exosites. To begin to address this, we first titrated a thrombin variant fluorescently labeled at exosite 1 with exosite 2 ligands, HD22 (a DNA aptamer), gamma'-peptide (an analog of the COOH terminus of the gamma'-chain of fibrinogen) or heparin. Concentration-dependent and saturable changes in fluorescence were elicited, supporting inter-exosite linkage. To explore the functional consequences of this phenomenon, we evaluated the capacity of exosite 2 ligands to inhibit thrombin binding to gamma(A)/gamma(A)-fibrin, an interaction mediated solely by exosite 1. When gamma(A)/gamma(A)-fibrinogen was clotted with thrombin in the presence of HD22, gamma'-peptide, or prothrombin fragment 2 there was a dose-dependent and saturable decrease in thrombin binding to the resultant fibrin clots. Furthermore, HD22 reduced the affinity of thrombin for gamma(A)/gamma(A)-fibrin 6-fold and accelerated the dissociation of thrombin from preformed gamma(A)/gamma(A)-fibrin clots. Similar responses were obtained when surface plasmon resonance was used to monitor the interaction of thrombin with gamma(A)/gamma(A)-fibrinogen or fibrin. There is bidirectional communication between the exosites, because exosite 1 ligands, HD1 (a DNA aptamer) or hirudin-(54-65) (an analog of the COOH terminus of hirudin), inhibited the exosite 2-mediated interaction of thrombin with immobilized gamma'-peptide. These findings provide evidence for long range allosteric linkage between exosites 1 and 2 on thrombin, revealing further complexity to the mechanisms of thrombin regulation.
Figures



References
-
- Lane D. A., Philippou H., Huntington J. A. (2005) Blood 106, 2605–2612 - PubMed
-
- Meh D. A., Siebenlist K. R., Mosesson M. W. (1996) J. Biol. Chem. 271, 23121–23125 - PubMed
-
- Myles T., Yun T. H., Hall S. W., Leung L. L. (2001) J. Biol. Chem. 276, 25143–25149 - PubMed
-
- Esmon C. T., Lollar P. (1996) J. Biol. Chem. 271, 13882–13887 - PubMed
-
- Ayala Y. M., Cantwell A. M., Rose T., Bush L. A., Arosio D., Di Cera E. (2001) Proteins 45, 107–116 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

