Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation
- PMID: 19589923
- PMCID: PMC2756199
- DOI: 10.1182/blood-2009-01-199216
Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation
Abstract
Retroviral transduction of tumor antigen-specific T-cell receptor (TCR) genes into lymphocytes redirects T cells to lyse tumors. Furthermore, adoptive transfer of these lymphocytes has mediated objective responses in patients with metastatic cancer. From 2004 to 2006, more than 40 patients were treated with autologous gene-modified lymphocytes expressing a melanoma antigen-specific TCR at the National Cancer Institute. Eighteen such patients were analyzed for persistence and gene expression in vivo. In addition, the impact of epigenetic silencing and of lymphocyte restimulation was studied. Although gene-modified lymphocytes persisted in vivo, the shutdown of TCR transgene expression was observed. Bisulfite sequencing analysis and ex vivo DNA methyltransferase inhibition demonstrated that the decrease in gene expression did not result from DNA methylation. Surprisingly, down-regulation of vector-driven transgene transcriptional activity was not vector specific but mimicked that of endogenous genes. The decrease in TCR transgene expression, however, was reversed upon lymphocyte stimulation. These data demonstrate a lack of gamma-retroviral promoter-specific gene silencing in adoptively transferred human lymphocytes and support that transgene expression is largely affected by global cellular mechanisms. The use of immunomodulatory adjuvants, eg, vaccination or cytokine therapy, for in vivo T-cell activation may help overcome this metabolic quiescence and thus augment cellular immunotherapy-based cancer therapy.
Figures
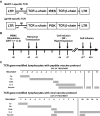


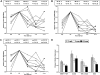
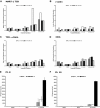
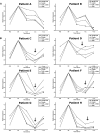
 ).
).Comment in
-
The hidden (and lazy) TCR.Blood. 2009 Oct 1;114(14):2855-6. doi: 10.1182/blood-2009-07-234153. Blood. 2009. PMID: 19797529 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

