Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice
- PMID: 19589945
- PMCID: PMC2739827
- DOI: 10.1152/ajpgi.90446.2008
Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice
Abstract
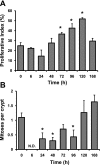
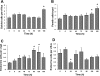
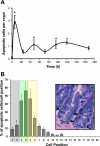
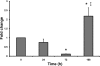
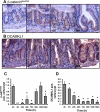
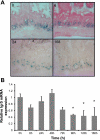
The intestinal epithelium is in a constant state of renewal. The rapid turnover of cells is fed by a hierarchy of transit amplifying and stem/progenitor cells destined to give rise to the four differentiated epithelial lineages of the small intestine. Doxorubicin (Dox) is a commonly used chemotherapeutic agent that preferentially induces apoptosis in the intestinal stem cell zone (SCZ). We hypothesized that Dox treatment would initially decrease "+4" intestinal stem cell numbers with a subsequent expansion during mucosal repair. Temporal assessment following Dox treatment demonstrated rapid induction of apoptosis in the SCZ leading to a decrease in the number of intestinal stem/progenitor cells as determined by flow cytometry for CD45(-) SP cells, and immunohistochemistry of cells positive for putative +4 stem cell markers beta-cat(Ser552) and DCAMKL1. Between 96 and 168 h postinjection, overall proliferation in the crypts increased concomitant with increases in both absolute and relative numbers of goblet, Paneth, and enteroendocrine cells. This regeneration phase was also associated with increases of CD45(-) SP cells, beta-cat(Ser552)-positive cells, crypt fission, and crypt number. We used Lgr5-lacZ mice to assess behavior of Lgr5-positive stem cells following Dox and found no change in this cell population. Lgr5 mRNA level was also measured and showed no change immediately after Dox but decreased during the regeneration phase. Together these data suggest that, following Dox-induced injury, expansion of intestinal stem cells occurs during mucosal repair. On the basis of available markers this expansion appears to be predominantly the +4 stem cell population rather than those of the crypt base.
Figures




References
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1: 113–118, 2000. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. - PubMed
-
- Beck PL, Wong JF, Li Y, Swaminathan S, Xavier RJ, Devaney KL, Podolsky DK. Chemotherapy- and radiotherapy-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 126: 796–808, 2004. - PubMed
-
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am J Anat 160: 51–63, 1981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

