Modulation of alpha2-adrenoceptor functions by heterotrimeric Galphai protein isoforms
- PMID: 19589951
- PMCID: PMC2847768
- DOI: 10.1124/jpet.109.157230
Modulation of alpha2-adrenoceptor functions by heterotrimeric Galphai protein isoforms
Erratum in
- J Pharmacol Exp Ther. 2010 Apr;333(1):351. Birnbaumer, Lutz [added]
Abstract
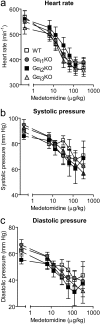
Subtype diversity of heterotrimeric G proteins and G protein-coupled receptors enables a wide spectrum of signal transduction. However, the significance of isoforms within receptor or G protein subfamilies has not been fully elucidated. In the present study, we have tested whether alpha(2)-adrenoceptors require specific Galpha isoforms for their function in vivo. In particular, we analyzed the role of the highly homologous Galpha(i) isoforms, Galpha(i1), Galpha(i2), and Galpha(i3), in typical alpha(2)-adrenoceptor-controlled functions. Mice with targeted deletions in the genes encoding Galpha(i1), Galpha(i2), or Galpha(i3) were used to test the effects of alpha(2)-adrenoceptor stimulation by the agonist medetomidine. The alpha(2)-adrenoceptor agonist medetomidine inhibited [(3)H]norepinephrine release from isolated prefrontal brain cortex or cardiac atria tissue specimens with similar potency and efficacy in tissues from wild-type or Galpha(i)-deficient mice. In vivo, bradycardia, hypotension, induction of sleep, antinociception, and hypothermia induced by alpha(2)-adrenoceptor activation did not differ between wild-type and Galpha(i)-knockout mice. However, the effects of the alpha(2)-agonists medetomidine or 5-bromo-6-(2-imidazolin-2-ylamino)quin-oxaline tartrate (UK14,304) on spontaneous locomotor activity or anesthetic sparing were reduced or absent, respectively, in mice lacking Galpha(i2). In microdissected locus coeruleus neurons or postganglionic sympathetic neurons from stellate ganglia, all three Galpha(i) subunits were expressed as determined by quantitative reverse transcription-polymerase chain reaction, with Galpha(i1) and Galpha(i2) dominating over Galpha(i3). Functional redundancy of the highly homologous Galpha(i) isoforms may predominate over specificity to regulate distinct intracellular pathways downstream of alpha(2)-adrenoceptors in vivo. In contrast, inhibition of locomotor activity and anesthetic sparing may be elicited by a specific coupling of alpha(2A)-adrenoceptors via the Galpha(i2) isoform to intracellular pathways.
Figures






References
-
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. ( 1999) Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol Pharmacol 56: 154–161 - PubMed
-
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L. ( 2002) Feedback inhibition of catecholamine release by two different α2-adrenoceptor subtypes prevents progression of heart failure. Circulation 106: 2491–2496 - PubMed
-
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. ( 1994) International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev 46: 121–136 - PubMed
-
- Gerhardt MA, Neubig RR. ( 1991) Multiple Gi protein subtypes regulate a single effector mechanism. Mol Pharmacol 40: 707–711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

