Dependence of mouse embryonic stem cells on threonine catabolism
- PMID: 19589965
- PMCID: PMC4373593
- DOI: 10.1126/science.1173288
Dependence of mouse embryonic stem cells on threonine catabolism
Abstract
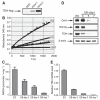
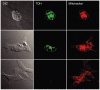
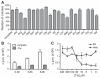
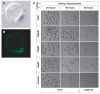
Measurements of the abundance of common metabolites in cultured embryonic stem (ES) cells revealed an unusual state with respect to one-carbon metabolism. These findings led to the discovery of copious expression of the gene encoding threonine dehydrogenase (TDH) in ES cells. TDH-mediated catabolism of threonine takes place in mitochondria to generate glycine and acetyl-coenzyme A (CoA), with glycine facilitating one-carbon metabolism via the glycine cleavage system and acetyl-CoA feeding the tricarboxylic acid cycle. Culture media individually deprived of each of the 20 amino acids were applied to ES cells, leading to the discovery that ES cells are critically dependent on one amino acid--threonine. These observations show that ES cells exist in a high-flux backbone metabolic state comparable to that of rapidly growing bacterial cells.
Figures


References
-
- Alberts B, et al. Molecular Biology of the Cell. ed. 5 Garland, New York: 2007. p. 1.
-
- Snow MHL. J. Embryol. Exp. Morphol. 1977;42:293.
-
- Smith AG. Annu. Rev. Cell Dev. Biol. 2001;17:435. - PubMed
-
- Tu BP, et al. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16886. - PubMed
-
- Warren L, Buchanan JM. J. Biol. Chem. 1957;229:613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

