Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue
- PMID: 19591192
- PMCID: PMC2753758
- DOI: 10.1002/bit.22440
Shear stress magnitude and duration modulates matrix composition and tensile mechanical properties in engineered cartilaginous tissue
Abstract
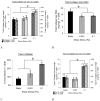
Cartilage tissue-engineering strategies aim to produce a functional extracellular matrix similar to that of the native tissue. However, none of the myriad approaches taken have successfully generated a construct possessing the structure, composition, and mechanical properties of healthy articular cartilage. One possible approach to modulating the matrix composition and mechanical properties of engineered tissues is through the use of bioreactor-driven mechanical stimulation. In this study, we hypothesized that exposing scaffold-free cartilaginous tissue constructs to 7 days of continuous shear stress at 0.001 or 0.1 Pa would increase collagen deposition and tensile mechanical properties compared to that of static controls. Histologically, type II collagen staining was evident in all construct groups, while a surface layer of type I collagen increased in thickness with increasing shear stress magnitude. The areal fraction of type I collagen was higher in the 0.1-Pa group (25.2 +/- 2.2%) than either the 0.001-Pa (13.6 +/- 3.8%) or the static (7.9 +/- 1.5%) group. Type II collagen content, as assessed by ELISA, was also higher in the 0.1-Pa group (7.5 +/- 2.1%) compared to the 0.001-Pa (3.0 +/- 2.25%) or static groups (3.7 +/- 3.2%). Temporal gene expression analysis showed a flow-induced increase in type I and type II collagen expression within 24 h of exposure. Interestingly, while the 0.1-Pa group showed higher collagen content, this group retained less sulfated glycosaminoglycans in the matrix over time in bioreactor culture. Increases in both tensile Young's modulus and ultimate strength were observed with increasing shear stress, yielding constructs possessing a modulus of nearly 5 MPa and strength of 1.3 MPa. This study demonstrates that shear stress is a potent modulator of both the amount and type of synthesized extracellular matrix constituents in engineered cartilaginous tissue with corresponding effects on mechanical function.
Copyright 2009 Wiley Periodicals, Inc.
Figures



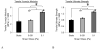
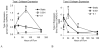
References
-
- Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop. 2001;(391 Suppl):S280–94. - PubMed
-
- Bae DK, Yoon KH, Song SJ. Cartilage healing after microfracture in osteoarthritic knees. Arthroscopy. 2006;22(4):367–74. - PubMed
-
- Berg RA. Determination of 3 and 4 hydroxyproline. In: Frederiksen LCaD., editor. Methods of Enzymology. New York: New York Academic Press; 1982. pp. 393–394. - PubMed
-
- Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G, Freed LE. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8(1):73–84. - PubMed
-
- Brandt KD, Doherty M, Lohmander LS. Osteoarthritis. New York: Oxford University Press; 1998. Composition and structure of articular cartilage; pp. 110–111.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

