Patent arterial duct
- PMID: 19591690
- PMCID: PMC2716300
- DOI: 10.1186/1750-1172-4-17
Patent arterial duct
Abstract
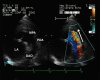
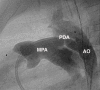
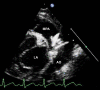
Patent arterial duct (PAD) is a congenital heart abnormality defined as persistent patency in term infants older than three months. Isolated PAD is found in around 1 in 2000 full term infants. A higher prevalence is found in preterm infants, especially those with low birth weight. The female to male ratio is 2:1. Most patients are asymptomatic when the duct is small. With a moderate-to-large duct, a characteristic continuous heart murmur (loudest in the left upper chest or infraclavicular area) is typical. The precordium may be hyperactive and peripheral pulses are bounding with a wide pulse pressure. Tachycardia, exertional dyspnoea, laboured breathing, fatigue or poor growth are common. Large shunts may lead to failure to thrive, recurrent infection of the upper respiratory tract and congestive heart failure. In the majority of cases of PAD there is no identifiable cause. Persistence of the duct is associated with chromosomal aberrations, asphyxia at birth, birth at high altitude and congenital rubella. Occasional cases are associated with specific genetic defects (trisomy 21 and 18, and the Rubinstein-Taybi and CHARGE syndromes). Familial occurrence of PAD is uncommon and the usual mechanism of inheritance is considered to be polygenic with a recurrence risk of 3%. Rare families with isolated PAD have been described in which the mode of inheritance appears to be dominant or recessive. Familial incidence of PAD has also been linked to Char syndrome, familial thoracic aortic aneurysm/dissection associated with patent arterial duct, and familial patent arterial duct and bicuspid aortic valve associated with hand abnormalities. Diagnosis is based on clinical examination and confirmed with transthoracic echocardiography. Assessment of ductal blood flow can be made using colour flow mapping and pulsed wave Doppler. Antenatal diagnosis is not possible, as PAD is a normal structure during antenatal life. Conditions with signs and symptoms of pulmonary overcirculation secondary to a left-to-right shunt must be excluded. Coronary, systemic and pulmonary arteriovenous fistula, peripheral pulmonary stenosis and ventricular septal defect with aortic regurgitation and collateral vessels must be differentiated from PAD on echocardiogram. In preterm infants with symptomatic heart failure secondary to PAD, treatment may be achieved by surgical ligation or with medical therapy blocking prostaglandin synthesis (indomethacin or ibuprofen). Transcatheter closure of the duct is usually indicated in older children. PAD in preterm and low birth weight infants is associated with significant co-morbidity and mortality due to haemodynamic instability. Asymptomatic patients with a small duct have a normal vital prognosis but have a lifetime risk of endocarditis. Patients with moderate-to-large ducts with significant haemodynamic alterations may develop irreversible changes to pulmonary vascularity and pulmonary hypertension.
Figures


References
-
- Benson LN, Cowan KN. The arterial duct: its persistence and its patency. In: Anderson RH, Baker EJ, Macartney FJ, Rigby ML, Shinebourne EA, Tynan M, editor. Paediatric Cardiology. second. Churchill Livingstone, London; 2002. pp. 1405–1459.
-
- Mullins CE, Pagotto L. Patent ductus arteriosus. In: Garson AJ, Bricker JT, Fisher DJ, Neish SR, editor. The Science and Practice of Pediatric Cardiology. Baltimore, Md: Williams & Wilkins; 1998. pp. 1181–1197.
-
- Mitchel S, Korones S, Berendes H. Congenital Heart Diseasein 56,109 births: incidence and naturel history. Circulation. 1971;43:323–332. - PubMed
-
- Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics. 1976;57:347–351. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

