Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins
- PMID: 19591848
- PMCID: PMC2784250
- DOI: 10.1016/j.mvr.2009.06.009
Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins
Abstract
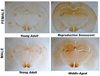
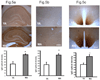
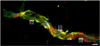
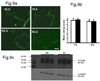
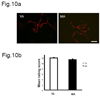
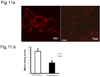
We previously demonstrated that there is a significantly greater transfer of intravenously-injected Evan's blue dye into the forebrain of acyclic (reproductive senescent) females compared to young adult females, indicating that blood brain barrier permeability is compromised in the reproductive senescent forebrain. The present study examined brain IgG expression and microvessel tight junction proteins to assess ovarian age-related changes in microvascular permeability, and further compared young and senescent females with age-matched males to distinguish changes attributable to age and reproductive senescence. Blood brain barrier breakdown are often associated with increased extravasation of plasma proteins and high levels of immunoglobulin G (IgG) in brain. In the present study, IgG expression was dramatically increased in the hippocampus and thalamus, but not the hypothalamus of reproductive senescent females compared to young adult females. In males, IgG expression was increased in all these regions in middle-aged animals (aged-matched to senescent females) as compared to young males (age-matched to the young adult females). Furthermore, the proportion of hippocampal microvessels with perivascular IgG immunoreactivity was significantly greater in reproductive senescent females as compared to young adult females, while middle-aged males and young adult males did not differ. The tight junctions between adjacent microvascular endothelial cells regulated by transmembrane proteins such as claudin-5 and occludin play a critical role in maintaining the blood brain barrier integrity. Increased hippocampal IgG expression in senescent females was paralleled by poor junctional localization of the tight junction protein claudin-5 in hippocampal microvessels. However, there was no difference in hippocampal claudin-5 localization between young adult and middle-aged males, indicating that dysregulation of this junctional protein was associated with ovarian aging. Parallel studies in human brain microvessels also revealed age-dependent disruption in claudin-5 distribution in post-menopausal women compared to pre-menopausal women. Collectively, these data support the hypothesis that constitutive loss of barrier integrity in the forebrain during reproductive senescence may be due, in part, to the selective loss of tight junction proteins in endothelial junctions.
Figures







References
-
- Aihara N, et al. Immunocytochemical localization of immunoglobulins in the rat brain: relationship to the blood-brain barrier. J Comp Neurol. 1994;342:481–496. - PubMed
-
- Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. - PubMed
-
- Andreeva AY, et al. Protein Kinase C Regulates the Phosphorylation and Cellular Localization of Occludin. J Biol Chem. 2001;276:38480–38486. - PubMed
-
- Arai K, et al. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. J Pharmacol. 2001;87:195–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

