Dexamethasone alters the hepatic inflammatory cellular profile without changes in matrix degradation during liver repair following biliary decompression
- PMID: 19592011
- PMCID: PMC2749887
- DOI: 10.1016/j.jss.2009.04.016
Dexamethasone alters the hepatic inflammatory cellular profile without changes in matrix degradation during liver repair following biliary decompression
Abstract
Background: Biliary atresia is characterized by extrahepatic bile duct obliteration along with persistent intrahepatic portal inflammation. Steroids are standard in the treatment of cholangitis following the Kasai portoenterostomy, and were advocated for continued suppression of the ongoing immunologic attack against intrahepatic ducts. Recent reports, however, have failed to demonstrate an improved patient outcome or difference in the need for liver transplant in postoperative patients treated with a variety of steroid regimes compared with historic controls. In the wake of progressive liver disease despite biliary decompression, steroids are hypothesized to suppress inflammation and promote bile flow without any supporting data regarding their effect on the emerging cellular and molecular mechanisms of liver repair. We have previously shown in a reversible model of cholestatic injury that repair is mediated by macrophages, neutrophils, and specific matrix metalloproteinase activity (MMP8); we questioned whether steroids would alter these intrinsic mechanisms.
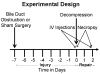
Methods: Rats underwent biliary ductal suspension for 7 d, followed by decompression. Rats were treated with IV dexamethasone or saline at the time of decompression. Liver tissue obtained at the time of decompression or after 2 d of repair was processed for morphometric analysis, immunohistochemistry, and quantitative RT-PCR.
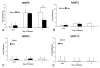
Results: There was a dramatic effect of dexamethasone on the inflammatory component with the initiation of repair. Immunohistochemistry revealed a reduction of both ED1+ hepatic macrophages and ED2+Kupffer cells in repair compared with saline controls. Dexamethasone treatment also reduced infiltrating neutrophils by day 2. TNF-alpha expression, increased during injury in both saline and dexamethasone groups, was markedly reduced by dexamethasone during repair (day 2) whereas IL-6, IL-10, and CINC-1 remained unchanged compared with saline controls. Dexamethasone reduced both MMP8 and TIMP1 expression by day 2, whereas MMP9, 13, and 14 were unchanged compared with sham controls. Despite substantial cellular and molecular changes during repair, collagen resorption was the same in both groups
Conclusion: Dexamethasone has clear effects on both the hepatic macrophage populations and infiltrating neutrophils following biliary decompression. Altered MMP and TIMP gene expression might suggest that steroids have the potential to modify matrix metabolism during repair. Nevertheless, successful resorption of collagen fibrosis proceeded presumably through other MMP activating mechanisms. We conclude that steroids do not impede the rapid intrinsic repair mechanisms of matrix degradation required for successful repair.
Figures




Similar articles
-
Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers.Am J Pathol. 2010 Mar;176(3):1271-81. doi: 10.2353/ajpath.2010.090527. Epub 2010 Jan 28. Am J Pathol. 2010. PMID: 20110408 Free PMC article.
-
Hepatic macrophages promote the neutrophil-dependent resolution of fibrosis in repairing cholestatic rat livers.Surgery. 2008 May;143(5):667-78. doi: 10.1016/j.surg.2008.01.008. Surgery. 2008. PMID: 18436015
-
Macrophage phenotype during cholestatic injury and repair: the persistent inflammatory response.J Pediatr Surg. 2001 Jan;36(1):220-8. doi: 10.1053/jpsu.2001.20059. J Pediatr Surg. 2001. PMID: 11150470
-
Biliary atresia: pathogenesis and treatment.Semin Liver Dis. 1998;18(3):281-93. doi: 10.1055/s-2007-1007164. Semin Liver Dis. 1998. PMID: 9773428 Review.
-
Recent progress in the etiopathogenesis of pediatric biliary disease, particularly Caroli's disease with congenital hepatic fibrosis and biliary atresia.Histol Histopathol. 2010 Feb;25(2):223-35. doi: 10.14670/HH-25.223. Histol Histopathol. 2010. PMID: 20017109 Review.
Cited by
-
Development and validation of a prediction model for cholangitis after percutaneous transhepatic cholangioscopic lithotripsy.Clin Exp Med. 2025 May 30;25(1):185. doi: 10.1007/s10238-025-01719-7. Clin Exp Med. 2025. PMID: 40445412 Free PMC article.
-
Dexamethasone pretreatment attenuates lung and kidney injury in cholestatic rats induced by hepatic ischemia/reperfusion.Inflammation. 2012 Feb;35(1):289-96. doi: 10.1007/s10753-011-9318-4. Inflammation. 2012. PMID: 21468628
-
Hepatocytes buried in the cirrhotic livers of patients with biliary atresia proliferate and function in the livers of urokinase-type plasminogen activator-NOG mice.Liver Transpl. 2014 Sep;20(9):1127-37. doi: 10.1002/lt.23916. Epub 2014 Aug 4. Liver Transpl. 2014. PMID: 24838399 Free PMC article.
-
Delphi Method Analysis and Consensus of Prevalent Distinctive Practices for Biliary Atresia Management after Kasai Portoenterostomy.J Indian Assoc Pediatr Surg. 2024 May-Jun;29(3):271-276. doi: 10.4103/jiaps.jiaps_250_23. Epub 2024 May 8. J Indian Assoc Pediatr Surg. 2024. PMID: 38912031 Free PMC article.
References
-
- Nio M, Ohi R. Biliary atresia. Semin Pediatr Surg. 2000;9:177–186. - PubMed
-
- Ahmed AF, Ohtani H, Nio M, Funaki N, Shimaoka S, Nagura H, Ohi R. CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol. 2001;193:383–389. - PubMed
-
- Ohya T, Fujimoto T, Shimomura H, Miyano T. Degeneration of intrahepatic bile duct with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg. 1995;30:515–518. - PubMed
-
- Haas JE. Bile duct and liver pathology in biliary atresia. World J Surg. 1978;2:561–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

