Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors
- PMID: 19592275
- PMCID: PMC2765864
- DOI: 10.1016/j.immuni.2009.04.018
Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors
Abstract
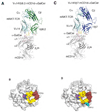
The semi-invariant natural killer T cell receptor (NKT TCR) recognizes CD1d-lipid antigens. Although the TCR alpha chain is typically invariant, the beta chain expression is more diverse, where three V beta chains are commonly expressed in mice. We report the structures of V alpha 14-V beta 8.2 and V alpha 14-V beta 7 NKT TCRs in complex with CD1d-alpha-galactosylceramide (alpha-GalCer) and the 2.5 A structure of the human NKT TCR-CD1d-alpha-GalCer complex. Both V beta 8.2 and V beta 7 NKT TCRs and the human NKT TCR ligated CD1d-alpha-GalCer in a similar manner, highlighting the evolutionarily conserved interaction. However, differences within the V beta domains of the V beta 8.2 and V beta 7 NKT TCR-CD1d complexes resulted in altered TCR beta-CD1d-mediated contacts and modulated recognition mediated by the invariant alpha chain. Mutagenesis studies revealed the differing contributions of V beta 8.2 and V beta 7 residues within the CDR2 beta loop in mediating contacts with CD1d. Collectively we provide a structural basis for the differential NKT TCR V beta usage in NKT cells.
Figures
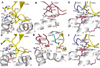




Similar articles
-
NKT TCR recognition of CD1d-α-C-galactosylceramide.J Immunol. 2011 Nov 1;187(9):4705-13. doi: 10.4049/jimmunol.1100794. Epub 2011 Sep 30. J Immunol. 2011. PMID: 21964029 Free PMC article.
-
Atypical natural killer T-cell receptor recognition of CD1d-lipid antigens.Nat Commun. 2016 Feb 15;7:10570. doi: 10.1038/ncomms10570. Nat Commun. 2016. PMID: 26875526 Free PMC article.
-
CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor.Nature. 2007 Jul 5;448(7149):44-9. doi: 10.1038/nature05907. Epub 2007 Jun 20. Nature. 2007. PMID: 17581592
-
Antigen specificity of semi-invariant CD1d-restricted T cell receptors: the best of both worlds?Immunol Cell Biol. 2004 Jun;82(3):285-94. doi: 10.1111/j.0818-9641.2004.01257.x. Immunol Cell Biol. 2004. PMID: 15186260 Review.
-
Tailored design of NKT-stimulatory glycolipids for polarization of immune responses.J Biomed Sci. 2017 Mar 23;24(1):22. doi: 10.1186/s12929-017-0325-0. J Biomed Sci. 2017. PMID: 28335781 Free PMC article. Review.
Cited by
-
Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor.Nat Immunol. 2012 Sep;13(9):857-63. doi: 10.1038/ni.2372. Epub 2012 Jul 22. Nat Immunol. 2012. PMID: 22820603
-
Structural basis of NKT cell inhibition using the T-cell receptor-blocking anti-CD1d antibody 1B1.J Biol Chem. 2019 Aug 30;294(35):12947-12956. doi: 10.1074/jbc.RA119.009403. Epub 2019 Jul 11. J Biol Chem. 2019. PMID: 31296659 Free PMC article.
-
Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells.PLoS Biol. 2011 Nov;9(11):e1001189. doi: 10.1371/journal.pbio.1001189. Epub 2011 Nov 1. PLoS Biol. 2011. PMID: 22069376 Free PMC article.
-
A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells.Immunity. 2011 Mar 25;34(3):327-39. doi: 10.1016/j.immuni.2011.02.001. Epub 2011 Mar 3. Immunity. 2011. PMID: 21376639 Free PMC article.
-
Structural and functional characterization of a novel nonglycosidic type I NKT agonist with immunomodulatory properties.J Immunol. 2012 Mar 1;188(5):2254-65. doi: 10.4049/jimmunol.1103049. Epub 2012 Feb 1. J Immunol. 2012. PMID: 22301545 Free PMC article.
References
-
- Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. - PubMed
-
- Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, Gumperz JE. Conserved and Heterogeneous Lipid Antigen Specificities of CD1d-Restricted NKT Cell Receptors. J Immunol. 2006;176:3625–3634. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

