Cell polarity factor Par3 binds SPTLC1 and modulates monocyte serine palmitoyltransferase activity and chemotaxis
- PMID: 19592499
- PMCID: PMC2757191
- DOI: 10.1074/jbc.M109.014365
Cell polarity factor Par3 binds SPTLC1 and modulates monocyte serine palmitoyltransferase activity and chemotaxis
Abstract
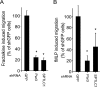
Elevated sphingolipids have been associated with increased cardiovascular disease. Conversely, atherosclerosis is reduced in mice by blocking de novo synthesis of sphingolipids catalyzed by serine palmitoyltransferase (SPT). The SPT enzyme is composed of the SPTLC1 and -2 subunits, and here we describe a novel protein-protein interaction between SPTLC1 and the PDZ protein Par3 (partitioning defective protein 3). Mammalian SPTLC1 orthologs have a highly conserved C terminus that conforms to a type II PDZ protein interaction motif, and by screening PDZ domain protein arrays with an SPTLC1 C-terminal peptide, we found it bound the third PDZ domain of Par3. Overlay and immunoprecipitation assays confirmed this interaction and indicate Par3 is able to associate with the SPTLC1/2 holoenzyme by binding the C-terminal SPTLC1 PDZ motif. The physiologic existence of the SPTLC1/2-Par3 complex was detected in mouse liver and macrophages, and short interfering RNA inhibition of Par3 in human THP-1 monocytes significantly reduced SPT activity and de novo ceramide synthesis by nearly 40%. Given monocyte recruitment into inflamed vessels is thought to promote atherosclerosis, and because Par3 and sphingolipids have been associated with polarized cell migration, we tested whether the ability of THP-1 monocytes to migrate toward MCP-1 (monocyte chemoattractant protein 1) depended upon Par3 and SPTLC1 expression. Knockdown of Par3 significantly reduced MCP1-induced chemotaxis of THP-1 monocytes, as did knockdown of SPTLC1, and this Par3 effect depended upon SPT activity and was blunted by ceramide treatment. In conclusion, protein arrays were used to identify a novel SPTLC1-Par3 interaction that associates with increased monocyte serine palmitoyltransferase activity and chemotaxis toward inflammatory signals.
Figures



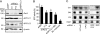

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

