ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells
- PMID: 19592505
- PMCID: PMC2708977
- DOI: 10.1073/pnas.0905931106
ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells
Abstract
Chromosomal rearrangements involving erythroblast transformation specific (ETS) family transcription factors were recently defined as the most common genetic alterations in human prostate cancer. Despite their prevalence, it is unclear what quantitative role they play in either initiation or progression of the disease. Using a lentiviral transduction and dissociated cell prostate regeneration approach, we find that acutely increased expression of ETS proteins in adult murine prostate epithelial cells is sufficient to induce the formation of epithelial hyperplasia and focal prostatic intraepithelial neoplasia (PIN) lesions, but not progression to carcinoma. However, combined expression of ERG with additional genetic alternations associated with human prostate cancer can lead to aggressive disease. Although ERG overexpression does not cooperate with loss of the tumor suppressor p53, it does collaborate with alterations in PI3K signaling, such as Pten knockdown or AKT up-regulation, to produce a well-differentiated adenocarcinoma. Most striking is our finding that overexpression of androgen receptor (AR) does not give rise to any hyperplastic lesions, but when combined with high levels of ERG, it promotes the development of a more poorly differentiated, invasive adenocarcinoma. These findings suggest that in human prostate cancer, the most potent function of ETS gene fusions may be to synergize with alternative genetic events and provide different pathways for carcinoma production and invasive behavior. Our results provide direct evidence for selective cooperating events in ERG-induced prostate tumorigenesis and offer a rational basis for combined therapeutic interventions against multiple oncogenic pathways in prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
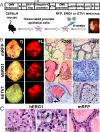

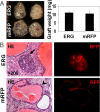
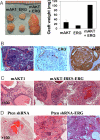

References
-
- Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. 1993;9:138–141. - PubMed
-
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. - PubMed
-
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–2434. - PubMed
-
- Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. - PubMed
-
- Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Evidence of recurrent gene fusions in common epithelial tumors. Trends Mol Med. 2006;12:529–536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

