Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport
- PMID: 19592509
- PMCID: PMC2718351
- DOI: 10.1073/pnas.0903328106
Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport
Abstract
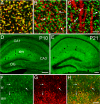
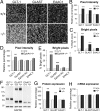
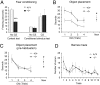
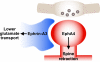
Increasing evidence indicates the importance of neuron-glia communication for synaptic function, but the mechanisms involved are not fully understood. We reported that the EphA4 receptor tyrosine kinase is in dendritic spines of pyramidal neurons of the adult hippocampus and regulates spine morphology. We now show that the ephrin-A3 ligand, which is located in the perisynaptic processes of astrocytes, is essential for maintaining EphA4 activation and normal spine morphology in vivo. Ephrin-A3-knockout mice have spine irregularities similar to those observed in EphA4-knockout mice. Remarkably, loss of ephrin-A3 or EphA4 increases the expression of glial glutamate transporters. Consistent with this, glutamate transport is elevated in ephrin-A3-null hippocampal slices whereas Eph-dependent stimulation of ephrin-A3 signaling inhibits glutamate transport. Furthermore, some forms of hippocampus-dependent learning are impaired in the ephrin-A3-knockout mice. Our results suggest that the interaction between neuronal EphA4 and glial ephrin-A3 bidirectionally controls synapse morphology and glial glutamate transport, ultimately regulating hippocampal function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

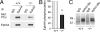

References
-
- Allen NJ, Barres BA. Signaling between glia and neurons: Focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. - PubMed
-
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. - PubMed
-
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. - PubMed
-
- Tanaka K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

