Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1
- PMID: 19592593
- PMCID: PMC2747905
- DOI: 10.1128/JB.00460-09
Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1
Abstract
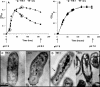
Sedimentary hopanes are pentacyclic triterpenoids that serve as biomarker proxies for bacteria and certain bacterial metabolisms, such as oxygenic photosynthesis and aerobic methanotrophy. Their parent molecules, the bacteriohopanepolyols (BHPs), have been hypothesized to be the bacterial equivalent of sterols. However, the actual function of BHPs in bacterial cells is poorly understood. Here, we report the physiological study of a mutant in Rhodopseudomonas palustris TIE-1 that is unable to produce any hopanoids. The deletion of the gene encoding the squalene-hopene cyclase protein (Shc), which cyclizes squalene to the basic hopene structure, resulted in a strain that no longer produced any polycyclic triterpenoids. This strain was able to grow chemoheterotrophically, photoheterotrophically, and photoautotrophically, demonstrating that hopanoids are not required for growth under normal conditions. A severe growth defect, as well as significant morphological damage, was observed when cells were grown under acidic and alkaline conditions. Although minimal changes in shc transcript expression were observed under certain conditions of pH shock, the total amount of hopanoid production was unaffected; however, the abundance of methylated hopanoids significantly increased. This suggests that hopanoids may play an indirect role in pH homeostasis, with certain hopanoid derivatives being of particular importance.
Figures






References
-
- Begley, M., C. G. Gahan, and C. Hill. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625-651. - PubMed
-
- Benz, R., D. Hallmann, K. Poralla, and H. Eibl. 1983. Interaction of hopanoids with phosphatidylcholines containing oleic and omega cyclohexyldodecanoic acid in lipid bilayer membranes. Chem. Phys. Lipids 34:7-24. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

