A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells
- PMID: 19592614
- PMCID: PMC2755982
- DOI: 10.1152/ajpheart.00374.2009
A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells
Abstract
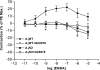
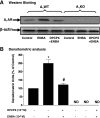
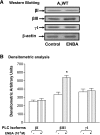
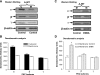
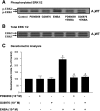
The A(1) adenosine receptor (A(1)AR) is coupled to G(i)/G(o) proteins, but the downstream signaling pathways in smooth muscle cells are unclear. This study was performed in coronary artery smooth muscle cells (CASMCs) isolated from the mouse heart [A(1)AR wild type (A(1)WT) and A(1)AR knockout (A(1)KO)] to delineate A(1)AR signaling through the PKC pathway. In A(1)WT cells, treatment with (2S)-N(6)-(2-endo-norbornyl)adenosine (ENBA; 10(-5)M) increased A(1)AR expression by 150%, which was inhibited significantly by the A(1)AR antagonist 1,3-dipropyl-8-cyclopentylxanthine (10(-6)M), but not in A(1)KO CASMCs. PKC isoforms were identified by Western blot analysis in the cytosolic and membrane fractions of cell homogenates of CASMCs. In A(1)WT and A(1)KO cells, significant levels of basal PKC-alpha were detected in the cytosolic fraction. Treatment with the A(1)AR agonist ENBA (10(-5)M) translocated PKC-alpha from the cytosolic to membrane fraction significantly in A(1)WT but not A(1)KO cells. Phospholipase C isoforms (betaI, betaIII, and gamma(1)) were analyzed using specific antibodies where ENBA treatment led to the increased expression of PLC-betaIII in A(1)WT CASMCs while having no effect in A(1)KO CASMCs. In A(1)WT cells, ENBA increased PKC-alpha expression and p42/p44 MAPK (ERK1/2) phospohorylation by 135% and 145%, respectively. These effects of ENBA were blocked by Gö-6976 (PKC-alpha inhibitor) and PD-98059 (p42/p44 MAPK inhibitor). We conclude that A(1)AR stimulation by ENBA activates the PKC-alpha signaling pathway, leading to p42/p44 MAPK phosphorylation in CASMCs.
Figures



References
-
- Ansari HR, Husain S, Abdel-Latif AA. Activation of p42/p44 mitogen-activated protein kinase and contraction by prostaglandin F2alpha, ionomycin, and thapsigargin in cat iris sphincter smooth muscle: inhibition by PD98059, KN-93, and isoproterenol. J Pharmacol Exp Ther 299: 178–186, 2001. - PubMed
-
- Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol 293: H3448–H3455, 2007. - PubMed
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. - PubMed
-
- Ding Y, Schwartz D, Posner P, Zhong J. Hypotonic swelling stimulates L-type Ca2+ channel activity in vascular smooth muscle cells through PKC. Am J Physiol Cell Physiol 287: C413–C421, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

