IL-6 is required for airway mucus production induced by inhaled fungal allergens
- PMID: 19592651
- PMCID: PMC2929571
- DOI: 10.4049/jimmunol.0802923
IL-6 is required for airway mucus production induced by inhaled fungal allergens
Abstract
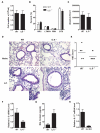
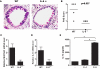
Allergic asthma is caused by inhaled allergens and is characterized by airway eosinophilia, as well as mucus hypersecretion, which can lead to airflow obstruction. Despite the association of increased IL-6 levels with human atopic asthma, the contribution of IL-6 to the development of allergic airway inflammation triggered by inhaled allergens remains unclear. In this study, we examined the role of IL-6 in a mouse model of allergic airway inflammation induced by direct airway exposure to extracts of Aspergillus fumigatus, a common allergen in humans. We show that inhaled A. fumigatus extracts rapidly trigger the production of IL-6 in the airways. IL-6 appears to be dispensable for the recruitment of eosinophils to the lung during the development of allergic airway inflammation. However, IL-6 is essential for mucus hypersecretion by airway epithelial cells triggered in response to inhaled A. fumigatus Ags. Impaired mucus production caused by IL-6 deficiency correlates with a severe reduction in the levels of IL-13, a major inducer of mucin glycoproteins. Thus, IL-6 is a key regulator of specific hallmark features of allergic airway inflammation and it could be a potential target for pulmonary diseases that are associated with goblet cell metaplasia and mucus hypersecretion.
Figures



References
-
- Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. - PubMed
-
- Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:804–815. - PubMed
-
- Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–2729. - PubMed
-
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

