Specific transduction of HIV-susceptible cells for CCR5 knockdown and resistance to HIV infection: a novel method for targeted gene therapy and intracellular immunization
- PMID: 19593160
- PMCID: PMC3777721
- DOI: 10.1097/QAI.0b013e3181b010a0
Specific transduction of HIV-susceptible cells for CCR5 knockdown and resistance to HIV infection: a novel method for targeted gene therapy and intracellular immunization
Abstract
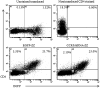
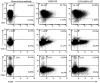
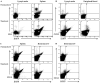
HIV-1 gene therapy offers a promising alternative to small molecule antiretroviral treatments and current vaccination strategies by transferring, into HIV-1-susceptible cells, the genetic ability to resist infection. The need for novel and innovative strategies to prevent and treat HIV-1 infection is critical due to devastating effects of the virus in developing countries, high cost, toxicity, generation of escape mutants from antiretroviral therapies, and the failure of past and current vaccination efforts. As a first step toward achieving this goal, an HIV-1-susceptible cell-specific targeting vector was evaluated to selectively transfer, into CCR5-positive target cells, an anti-HIV CCR5 shRNA gene for subsequent knockdown of CCR5 expression and protection from HIV-1 infection. Using a ZZ domain/monoclonal antibody-conjugated Sindbis virus glycoprotein pseudotyped lentiviral vector, here we demonstrate the utility of this strategy for HIV-1 gene therapy by specifically targeting HIV-1-susceptible cells and engineering these cells to resist HIV-1 infection. CCR5-positive human cells were successfully and specifically targeted in vitro and in vivo for transduction by a lentiviral vector expressing a highly potent CCR5 shRNA which conferred resistance to HIV-1 infection. Here we report the initial evaluation of this targeting vector for HIV-1 gene therapy in a preexposure prophylactic setting.
Figures



References
-
- Winters MA, Baxter JD, Mayers DL, et al. Frequency of antiretroviral drug resistance mutations in HIV-1 strains from patients failing triple drug regimens. The Terry Beirn Community Programs for Clinical Research on AIDS. Antivir Ther. 2000;5:57–63. - PubMed
-
- Lafeuillade A, Poggi C, Hittinger G, et al. Phenotypic and genotypic resistance to nucleoside reverse transcriptase inhibitors in HIV-1 clinical isolates. HIV Med. 2002;2:231–235. - PubMed
-
- Marks K, Gulick RM. New antiretroviral agents for the treatment of HIV infection. Curr HIV/AIDS Rep. 2004;1:82–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

