The Kunitz-like modulatory protein haemangin is vital for hard tick blood-feeding success
- PMID: 19593376
- PMCID: PMC2701603
- DOI: 10.1371/journal.ppat.1000497
The Kunitz-like modulatory protein haemangin is vital for hard tick blood-feeding success
Abstract
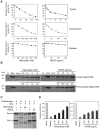
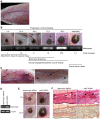
Ticks are serious haematophagus arthropod pests and are only second to mosquitoes as vectors of diseases of humans and animals. The salivary glands of the slower feeding hard ticks such as Haemaphysalis longicornis are a rich source of bioactive molecules and are critical to their biologic success, yet distinct molecules that help prolong parasitism on robust mammalian hosts and achieve blood-meals remain unidentified. Here, we report on the molecular and biochemical features and precise functions of a novel Kunitz inhibitor from H. longicornis salivary glands, termed Haemangin, in the modulation of angiogenesis and in persistent blood-feeding. Haemangin was shown to disrupt angiogenesis and wound healing via inhibition of vascular endothelial cell proliferation and induction of apoptosis. Further, this compound potently inactivated trypsin, chymotrypsin, and plasmin, indicating its antiproteolytic potential on angiogenic cascades. Analysis of Haemangin-specific gene expression kinetics at different blood-feeding stages of adult ticks revealed a dramatic up-regulation prior to complete feeding, which appears to be functionally linked to the acquisition of blood-meals. Notably, disruption of Haemangin-specific mRNA by a reverse genetic tool significantly diminished engorgement of adult H. longicornis, while the knock-down ticks failed to impair angiogenesis in vivo. To our knowledge, we have provided the first insights into transcriptional responses of human microvascular endothelial cells to Haemangin. DNA microarray data revealed that Haemangin altered the expression of 3,267 genes, including those of angiogenic significance, further substantiating the antiangiogenic function of Haemangin. We establish the vital roles of Haemangin in the hard tick blood-feeding process. Moreover, our results provide novel insights into the blood-feeding strategies that enable hard ticks to persistently feed and ensure full blood-meals through the modulation of angiogenesis and wound healing processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nature Med. 1998;4:336–340. - PubMed
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995;1:27–31. - PubMed
-
- D'Amore PA, Thompson RW. Mechanisms of angiogenesis. Annu Rev Physiol. 1987;49:453–464. - PubMed
-
- van Hinsbergh VWM, Engelse MA, Quax PHA. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vas Biol. 2006;26:716–728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

