Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells
- PMID: 19593636
- PMCID: PMC3633461
- DOI: 10.1007/s10549-009-0457-3
Antimitotic chemotherapeutics promote adhesive responses in detached and circulating tumor cells
Abstract
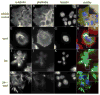
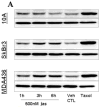
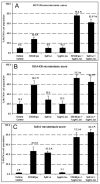
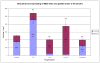
In the clinical treatment of breast cancer, antimitotic cytotoxic agents are one of the most commonly employed chemotherapies, owing largely to their antiproliferative effects on the growth and survival of adherent cells in studies that model primary tumor growth. Importantly, the manner in which these chemotherapeutics impact the metastatic process remains unclear. Furthermore, since dissemination of tumor cells through the systemic circulation and lymphatics necessitates periods of detached survival, it is equally important to consider how circulating tumor cells respond to such compounds. To address this question, we exposed both nontumorigenic and tumor-derived epithelial cell lines to two antitumor compounds, jasplakinolide and paclitaxel (Taxol), in a series of attached and detached states. We report here that jasplakinolide promoted the extension of microtubule-based projections and microtentacle protrusions in adherent and suspended cells, respectively. These protrusions were specifically enriched by upregulation of a stable post-translationally modified form of alpha-tubulin, and this occurred prior to, and independently of any reductions in cellular viability. Microtubule stabilization with Taxol significantly enhanced these effects. Additionally, Taxol promoted the attachment and spreading of suspended tumor cell populations on extracellular matrix. While the antiproliferative effects of these compounds are well recognized and clinically valuable, our findings that microfilament and microtubule binding chemotherapeutics rapidly increase the mechanisms that promote endothelial adhesion of circulating tumor cells warrant caution to avoid inadvertently enhancing metastatic potential, while targeting cell division.
Figures




References
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. - PubMed
-
- Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J Biol Chem. 2000;275:22607–10. - PubMed
-
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007:re5. - PubMed
-
- Weber K, Lazarides E, Goldman RD, Vogel A, Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

