Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice
- PMID: 19594304
- PMCID: PMC3153379
- DOI: 10.1359/jbmr.090730
Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice
Abstract
Intermittent parathyroid hormone (PTH) treatment is a potent bone anabolic principle that suppresses expression of the bone formation inhibitor Sost. We addressed the relevance of Sost suppression for PTH-induced bone anabolism in vivo using mice with altered Sost gene dosage. Six-month-old Sost overexpressing and 2-month-old Sost deficient male mice and their wild-type littermates were subjected to daily injections of 100 microg/kg PTH(1-34) or vehicle for a 2-month period. A follow-up study was performed in Sost deficient mice using 40 and 80 microg/kg PTH(1-34). Animals were sacrificed 4 hours after the final PTH administration and Sost expression in long bone diaphyses was determined by qPCR. Bone changes were analyzed in vivo in the distal femur metaphysis by pQCT and ex vivo in the tibia and lumbar spine by DXA. Detailed ex vivo analyses of the femur were performed by pQCT, microCT, and histomorphometry. Overexpression of Sost resulted in osteopenia and Sost deletion in high bone mass. As shown before, PTH suppressed Sost in wild-type mice. PTH treatment induced substantial increases in bone mineral density, content, and cortical thickness and in aging wild-type mice also led to cancellous bone gain owing to amplified bone formation rates. PTH-induced bone gain was blunted at all doses and skeletal sites in Sost overexpressing and deficient mice owing to attenuated bone formation rates, whereas bone resorption was not different from that in PTH-treated wild-type controls. These data suggest that suppression of the bone formation inhibitor Sost by intermittent PTH treatment contributes to PTH bone anabolism.
Copyright 2010 American Society for Bone and Mineral Research.
Figures
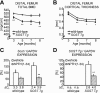
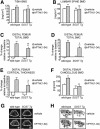
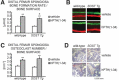
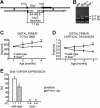



Comment in
-
Building bone with a SOST-PTH partnership.J Bone Miner Res. 2010 Feb;25(2):175-7. doi: 10.1002/jbmr.53. J Bone Miner Res. 2010. PMID: 20175218 No abstract available.
References
-
- Bonnick SL. Osteoporosis in men and women. Clin Cornerstone. 2006;8:28–39. - PubMed
-
- Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. - PubMed
-
- Juppner H, Abou-Samra AB, Freeman M, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. - PubMed
-
- Nishida S, Yamaguchi A, Tanizawa T, et al. Increased bone formation by intermittent parathyroid hormone administration is due to the stimulation of proliferation and differentiation of osteoprogenitor cells in bone marrow. Bone. 1994;15:717–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

