Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits
- PMID: 19594751
- PMCID: PMC2765319
- DOI: 10.1111/j.1476-5381.2009.00321.x
Salicylate, an aspirin metabolite, specifically inhibits the current mediated by glycine receptors containing alpha1-subunits
Abstract
Background and purpose: Aspirin or its metabolite sodium salicylate is widely prescribed and has many side effects. Previous studies suggest that targeting neuronal receptors/ion channels is one of the pathways by which salicylate causes side effects in the nervous system. The present study aimed to investigate the functional action of salicylate on glycine receptors at a molecular level.
Experimental approach: Whole-cell patch-clamp and site-directed mutagenesis were deployed to examine the effects of salicylate on the currents mediated by native glycine receptors in cultured neurones of rat inferior colliculus and by glycine receptors expressed in HEK293T cells.
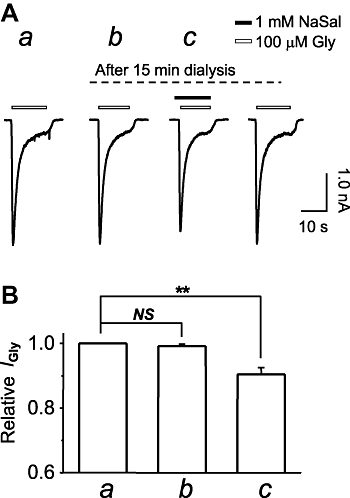
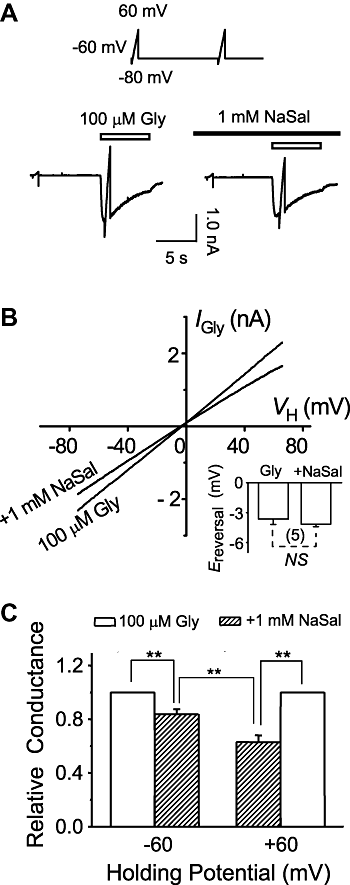
Key results: Salicylate effectively inhibited the maximal current mediated by native glycine receptors without altering the EC(50) and the Hill coefficient, demonstrating a non-competitive action of salicylate. Only when applied simultaneously with glycine and extracellularly, could salicylate produce this antagonism. In HEK293T cells transfected with either alpha1-, alpha2-, alpha3-, alpha1beta-, alpha2beta- or alpha3beta-glycine receptors, salicylate only inhibited the current mediated by those receptors that contained the alpha1-subunit. A single site mutation of I240V in the alpha1-subunit abolished inhibition by salicylate.
Conclusions and implications: Salicylate is a non-competitive antagonist specifically on glycine receptors containing alpha1-subunits. This action critically involves the isoleucine-240 in the first transmembrane segment of the alpha1-subunit. Our findings may increase our understanding of the receptors involved in the side effects of salicylate on the central nervous system, such as seizures and tinnitus.
Figures





Similar articles
-
Quercetin subunit specifically reduces GlyR-mediated current in rat hippocampal neurons.Neuroscience. 2007 Aug 24;148(2):548-59. doi: 10.1016/j.neuroscience.2007.06.007. Epub 2007 Jul 30. Neuroscience. 2007. PMID: 17664043
-
High doses of salicylate and aspirin are inhibitory on acid-sensing ion channels and protective against acidosis-induced neuronal injury in the rat cortical neuron.J Neurosci Res. 2012 Jan;90(1):267-77. doi: 10.1002/jnr.22742. Epub 2011 Oct 4. J Neurosci Res. 2012. PMID: 21969311
-
Effects of salicylate on transient outward and delayed rectifier potassium channels in rat inferior colliculus neurons.Neurosci Lett. 2004 Oct 14;369(2):115-20. doi: 10.1016/j.neulet.2004.07.037. Neurosci Lett. 2004. PMID: 15450679
-
Anti-inflammatory effects of aspirin and sodium salicylate.Eur J Pharmacol. 2002 Jun 28;447(1):1-9. doi: 10.1016/s0014-2999(02)01828-9. Eur J Pharmacol. 2002. PMID: 12106797 Review.
-
Pharmacokinetics of aspirin: evaluating shortcomings in the literature.Expert Opin Drug Metab Toxicol. 2024 Aug;20(8):727-740. doi: 10.1080/17425255.2024.2386368. Epub 2024 Aug 2. Expert Opin Drug Metab Toxicol. 2024. PMID: 39092921 Review.
Cited by
-
Tinnitus-related changes in the inferior colliculus.Front Neurol. 2015 Mar 30;6:61. doi: 10.3389/fneur.2015.00061. eCollection 2015. Front Neurol. 2015. PMID: 25870582 Free PMC article. Review.
-
Sodium salicylate suppresses GABAergic inhibitory activity in neurons of rodent dorsal raphe nucleus.PLoS One. 2015 May 11;10(5):e0126956. doi: 10.1371/journal.pone.0126956. eCollection 2015. PLoS One. 2015. PMID: 25962147 Free PMC article.
-
Behavioral and Immunohistochemical Evidence for Suppressive Effects of Goshajinkigan on Salicylate-Induced Tinnitus in Rats.Brain Sci. 2022 Apr 30;12(5):587. doi: 10.3390/brainsci12050587. Brain Sci. 2022. PMID: 35624974 Free PMC article.
-
Shared and Related Molecular Targets and Actions of Salicylic Acid in Plants and Humans.Cells. 2023 Jan 4;12(2):219. doi: 10.3390/cells12020219. Cells. 2023. PMID: 36672154 Free PMC article. Review.
-
Local Application of Sodium Salicylate Enhances Auditory Responses in the Rat's Dorsal Cortex of the Inferior Colliculus.Front Neurol. 2014 Nov 14;5:235. doi: 10.3389/fneur.2014.00235. eCollection 2014. Front Neurol. 2014. PMID: 25452744 Free PMC article.
References
-
- Argence M, Saez I, Sassu R, Vassias I, Vidal PP, de Waele C. Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: an in situ and immunofluorescence study. Neuroscience. 2006;141:1193–1207. - PubMed
-
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991;14:458–461. - PubMed
-
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

