PR3-ANCA in Wegener's granulomatosis prime human mononuclear cells for enhanced activation via TLRs and NOD1/2
- PMID: 19594951
- PMCID: PMC2717921
- DOI: 10.1186/1746-1596-4-23
PR3-ANCA in Wegener's granulomatosis prime human mononuclear cells for enhanced activation via TLRs and NOD1/2
Abstract
Background: Anti-neutrophil cytoplasmic antibodies (ANCA) is autoantibodies characteristic of vasculitis diseases. A connection between ANCA and Wegener's granulomatosis was well established. The interaction of both ANCA phenotypes (PR3-ANCA and MPO-ANCA) with leukocytes provoked cell activation, which might be involved in the pathogenesis of ANCA-related Wegener's granulomatosis.
Methods: In this study, we examined whether PR3-ANCA sera and purified immunoglobulins from patients with Wegener's granulomatosis prime human monocytic cells for enhanced responses to microbial components in terms of production of proinflammatory cytokines.
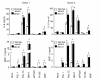
Results: Flow cytometry demonstrated that stimulation with antibodies to proteinase 3 enhanced the expression of TLR2, 3, 4, 7, and 9, NOD1, and NOD2 in human mononuclear cells. The sera and purified immunoglobulins significantly primed human mononuclear cells to secrete interleukin-8 in response to microbial components via TLRs and NODs. Priming effects were also observed for the production of interleukin-6, monocyte chemoattractant protein-1, and tumor necrosis factor-alpha. On the other hand, PR3-ANCA-negative sera from patients with polyarteritis nodosa which possibly related to MPO-ANCA and aortitis syndrome as well as control sera from a healthy volunteer did not have any priming effects on PBMCs.
Conclusion: In conclusion, PR3-ANCA prime human mononuclear cells to produce cytokines upon stimulation with various microbial components by up-regulating the TLR and NOD signaling pathway, and these mechanisms may partially participate in the inflammatory process in Wegener's granulomatosis.
Figures



References
-
- Hall JB, Wadham BM, Wood CJ, Ashton V, Adam WR. Vasculitis and glomerulonephritis: a subgroup with an antineutrophil cytoplasmic antibody. Aust N Z J Med. 1984;14:277–278. - PubMed
-
- Woude FJ van der, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, Giessen M van der, Hem GK van der, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–429. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

