Pediatric biobanks: approaching informed consent for continuing research after children grow up
- PMID: 19595370
- PMCID: PMC4779602
- DOI: 10.1016/j.jpeds.2009.04.034
Pediatric biobanks: approaching informed consent for continuing research after children grow up
Abstract
Objective: Proposals for pediatric biobanks have prompted questions of whether parental permission is sufficient to continue to use biological samples and data after the children become adults. The objective of this study was to examine adults' attitudes about continued research with their pediatric samples/data, particularly when they could not be located to provide consent.
Study design: Telephone interviews were conducted with 1186 patients from 5 academic medical centers by using a hypothetical scenario.
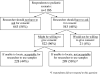
Results: Most respondents, 799 (67%), would not be concerned about the use of their sample/data after they reached adulthood. Those respondents who were concerned were more likely to be more private about their medical records, less trusting of medical researchers, or African-American. A total of 543 respondents (46%) believed their consent should be obtained to continue using their sample/data for research. Of these, 407 respondents (75%) would be at least moderately willing to give consent, when asked. Of the 1186 respondents, 310 (26%) would not want researchers to use their sample/data when they could not be located to ask for consent.
Conclusion: The data are consistent with the normative view that when feasible, adults should be asked for consent for continued research on their data collected during childhood, but it is generally acceptable to continue to conduct research when adults cannot be located. Further public engagement may help determine how best to balance the potential social value of continued research using pediatric samples/data and the expectations for explicit consent expressed by a minority of respondents that may reflect concerns about privacy and trust.
Figures
References
-
- Kaiser J. Genetics. US hospital launches large biobank of children's DNA. Science. 2006;312:1584–5. - PubMed
-
- Winickoff DE, Winickoff RN. The charitable trust as a model for genomic biobanks. N Engl J Med. 2003;349:1180–4. - PubMed
-
- Terry SF, Terry PF, Rauen KA, Uitto J, Bercovitch LG. Advocacy groups as research organizations: the PXE International example. Nat Rev Genet. 2007;8:157–64. - PubMed
-
- Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources