Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients
- PMID: 19595452
- PMCID: PMC2728798
- DOI: 10.1016/j.biomaterials.2009.06.042
Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients
Abstract
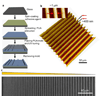
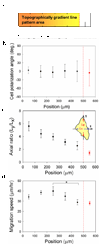
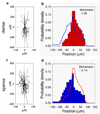
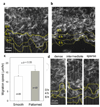
In this report, we describe using ultraviolet (UV)-assisted capillary force lithography (CFL) to create a model substratum of anisotropic micro- and nanotopographic pattern arrays with variable local density for the analysis of cell-substratum interactions. A single cell adhesion substratum with the constant ridge width (1 microm), and depth (400 nm) and variable groove widths (1-9.1 microm) allowed us to characterize the dependence of cellular responses, including cell shape, orientation, and migration, on the anisotropy and local density of the variable micro- and nanotopographic pattern. We found that fibroblasts adhering to the denser pattern areas aligned and elongated more strongly along the direction of ridges, vs. those on the sparser areas, exhibiting a biphasic dependence of the migration speed on the pattern density. In addition, cells responded to local variations in topography by altering morphology and migrating along the direction of grooves biased by the direction of pattern orientation (short term) and pattern density (long term), suggesting that single cells can sense the topography gradient. Molecular dynamic live cell imaging and immunocytochemical analysis of focal adhesions and actin cytoskeleton suggest that variable substratum topography can result in distinct types of cytoskeleton reorganization. We also demonstrate that fibroblasts cultured as monolayers on the same substratum retain most of the properties displayed by single cells. This result, in addition to demonstrating a more sophisticated method to study aspects of wound healing processes, strongly suggests that even in the presence of adhesive cell-cell interactions, the cues provided by the underlying substratum topography continue to exercise substantial influence on cell behavior. The described experimental platform might not only further our understanding of biomechanical regulation of cell-matrix interactions, but also contribute to bioengineering of devices with the optimally structured design of cell-material interface.
Figures



References
-
- Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–233. - PubMed
-
- Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. - PubMed
-
- Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, et al. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7(7):2122–2128. - PubMed
-
- Kim DH, Levchenko A, Suh KY. Engineered surface nanotopography for controlling cell-substrate interactions. In: Khademhosseini A JB, Takayama S, Toner M, editors. Micro- and Nanoengineering of the Cell Microenvironment: Technologies and Applications. Boston: Artech House; 2008. pp. 185–208.
-
- Ottani V, Raspanti M, Ruggeri A. Collagen structure and functional implications. Micron. 2001;32(3):251–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

