Engineering the binding properties of the T cell receptor:peptide:MHC ternary complex that governs T cell activity
- PMID: 19595460
- PMCID: PMC2773466
- DOI: 10.1016/j.molimm.2009.06.012
Engineering the binding properties of the T cell receptor:peptide:MHC ternary complex that governs T cell activity
Abstract
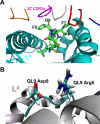
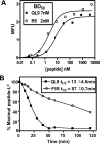
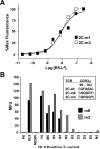
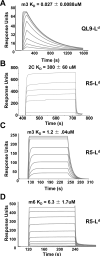
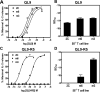
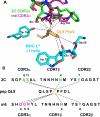
The potency of a T cell is determined in large part by two interactions, binding of a cognate peptide to the MHC, and binding of the T cell receptor (TCR) to this pepMHC. Various studies have attempted to assess the relative importance of these interactions, and to correlate the corresponding binding parameters with the level of T cell activity mediated by the peptide. To further examine the properties that govern optimal T cell activity, here we engineered both the peptide:MHC interaction and the TCR:pepMHC interaction to generate improved T cell activity. Using a system involving the 2C TCR and its allogeneic pepMHC ligand, QL9-L(d), we show that a peptide substitution of QL9 (F5R), increased the affinity and stability of the pep-L(d) complex (e.g. cell surface t(1/2)-values of 13 min for QL9-L(d) versus 87 min for F5R-L(d)). However, activity of peptide F5R for 2C T cells was not enhanced because the 2C TCR bound with very low affinity to F5R-L(d) compared to QL9-L(d) (K(D)=300 microM and K(D)=1.6 microM, respectively). To improve the affinity, yeast display of the 2C TCR was used to engineer two mutant TCRs that exhibited higher affinity for F5R-L(d) (K(D)=1.2 and 6.3 microM). T cells that expressed these higher affinity TCRs were stimulated by F5R-L(d) in the absence of CD8, and the highest affinity TCR exhibited enhanced activity for F5R compared to QL9. The results provide a guide to designing the explicit binding parameters that govern optimal T cell activities.
Figures
References
-
- Baker MB, Gagnon JS, Biddison EW, Wiley CD. Conversion of a T cell antagonist into an agonist by repairing a defect in the TCR/peptide/MHC interface: implications for TCR signaling. Immunity. 2000;13:475–84. - PubMed
-
- Chen JL, Stewart-Jones G, Bossi G, Lissin NM, Wooldridge L, Choi EM, Held G, Dunbar PR, Esnouf RM, Sami M, Boulter JM, Rizkallah P, Renner C, Sewell A, van der Merwe PA, Jakobsen BK, Griffiths G, Jones EY, Cerundolo V. Structural and kinetic basis for heightened immunogenicity of T cell vaccines. J Exp Med. 2005;201:1243–55. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

