Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae
- PMID: 19595690
- PMCID: PMC3277837
- DOI: 10.1016/j.ygcen.2009.07.006
Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in Drosophila larvae
Abstract
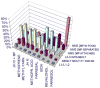
A long-unresolved question in the developmental biology of Drosophila melanogaster has been whether methyl farnesoid hormones secreted by the ring gland are necessary for larval maturation and metamorphosis. In this study, we have used RNAi techniques to inhibit 3-Hydroxy-3-Methylglutaryl CoA Reductase (HMGCR) expression selectively in the corpora allatal cells that produce the circulating farnesoid hormones. The developing larvae manifest a number of developmental, metabolic and morphogenetic derangements. These defects included the exhibition of an "ultraspiracle" death phenotype at the 1st to 2nd instar larval molt, similar to that exhibited by animals that are null for the farnesoid receptor ultraspiracle. The few larvae surviving past a second lethal period at the 2nd to 3rd instar larval molt, again with "ultraspiracle" phenotype, often became developmentally arrested after either attaining a misformed puparium or after formation of the white pupa. Survival past the "ultraspiracle" lethal phenotype could be rescued by dietary provision of an endogenous dedicated precursor to the three naturally secreted methyl farnesoid hormones. In addition to these developmental and morphogenetic defects, most larvae that survived to the late second instar exhibited a posterior-originating melanization of the tracheal system. These results support the hypothesis that larval methyl farnesoid hormones are necessary for larval survival and morphogenetic transformation through the larval and pupal metamorphic processes.
Figures




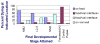

References
-
- Bellés X, Martín D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 2005;50:181–99. - PubMed
-
- Bernardo TJ, Dubrovskaya VA, Jannat H, Maughan B, Dubrovsky EB. Hormonal regulation of the E75 gene in Drosophila: identifying functional regulatory elements through computational and biological analysis. J Mol Biol. 2009;387:794–808. - PubMed
-
- Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

