Large-scale expansions of Friedreich's ataxia GAA repeats in yeast
- PMID: 19595718
- PMCID: PMC2722067
- DOI: 10.1016/j.molcel.2009.06.017
Large-scale expansions of Friedreich's ataxia GAA repeats in yeast
Abstract
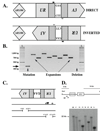
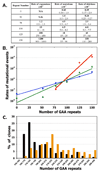
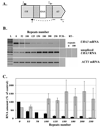
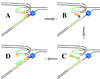
Large-scale expansions of DNA repeats are implicated in numerous hereditary disorders in humans. We describe a yeast experimental system to analyze large-scale expansions of triplet GAA repeats responsible for the human disease Friedreich's ataxia. When GAA repeats were placed into an intron of the chimeric URA3 gene, their expansions caused gene inactivation, which was detected on the selective media. We found that the rates of expansions of GAA repeats increased exponentially with their lengths. These rates were only mildly dependent on the repeat's orientation within the replicon, whereas the repeat-mediated replication fork stalling was exquisitely orientation dependent. Expansion rates were significantly elevated upon inactivation of the replication fork stabilizers, Tof1 and Csm3, but decreased in the knockouts of postreplication DNA repair proteins, Rad6 and Rad5, and the DNA helicase Sgs1. We propose a model for large-scale repeat expansions based on template switching during replication fork progression through repetitive DNA.
Figures


Similar articles
-
Replication stalling at Friedreich's ataxia (GAA)n repeats in vivo.Mol Cell Biol. 2004 Mar;24(6):2286-95. doi: 10.1128/MCB.24.6.2286-2295.2004. Mol Cell Biol. 2004. PMID: 14993268 Free PMC article.
-
Large-scale expansions of Friedreich's ataxia GAA•TTC repeats in an experimental human system: role of DNA replication and prevention by LNA-DNA oligonucleotides and PNA oligomers.Nucleic Acids Res. 2023 Sep 8;51(16):8532-8549. doi: 10.1093/nar/gkad441. Nucleic Acids Res. 2023. PMID: 37216608 Free PMC article.
-
Stalled DNA Replication Forks at the Endogenous GAA Repeats Drive Repeat Expansion in Friedreich's Ataxia Cells.Cell Rep. 2016 Aug 2;16(5):1218-1227. doi: 10.1016/j.celrep.2016.06.075. Epub 2016 Jul 14. Cell Rep. 2016. PMID: 27425605 Free PMC article.
-
Replication dependent and independent mechanisms of GAA repeat instability.DNA Repair (Amst). 2022 Oct;118:103385. doi: 10.1016/j.dnarep.2022.103385. Epub 2022 Aug 3. DNA Repair (Amst). 2022. PMID: 35952488 Free PMC article. Review.
-
Friedreich's ataxia: new insights.Emerg Top Life Sci. 2023 Dec 14;7(3):313-323. doi: 10.1042/ETLS20230017. Emerg Top Life Sci. 2023. PMID: 37698160 Review.
Cited by
-
Stimulation of gross chromosomal rearrangements by the human CEB1 and CEB25 minisatellites in Saccharomyces cerevisiae depends on G-quadruplexes or Cdc13.PLoS Genet. 2012;8(11):e1003033. doi: 10.1371/journal.pgen.1003033. Epub 2012 Nov 1. PLoS Genet. 2012. PMID: 23133402 Free PMC article.
-
Genetic Screens to Study GAA/TTC and Inverted Repeat Instability in Saccharomyces cerevisiae.Methods Mol Biol. 2020;2056:103-112. doi: 10.1007/978-1-4939-9784-8_6. Methods Mol Biol. 2020. PMID: 31586343 Free PMC article.
-
Cis- and Trans-Modifiers of Repeat Expansions: Blending Model Systems with Human Genetics.Trends Genet. 2018 Jun;34(6):448-465. doi: 10.1016/j.tig.2018.02.005. Epub 2018 Mar 19. Trends Genet. 2018. PMID: 29567336 Free PMC article. Review.
-
Alternative DNA Structures In Vivo: Molecular Evidence and Remaining Questions.Microbiol Mol Biol Rev. 2020 Dec 23;85(1):e00110-20. doi: 10.1128/MMBR.00110-20. Print 2021 Feb 17. Microbiol Mol Biol Rev. 2020. PMID: 33361270 Free PMC article. Review.
-
Replication-independent instability of Friedreich's ataxia GAA repeats during chronological aging.Proc Natl Acad Sci U S A. 2021 Feb 2;118(5):e2013080118. doi: 10.1073/pnas.2013080118. Proc Natl Acad Sci U S A. 2021. PMID: 33495349 Free PMC article.
References
-
- Andersen PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell research. 2008;18:162–173. - PubMed
-
- Anderson S, DePamphilis ML. Metabolism of Okazaki fragments during simian virus 40 DNA replication. J Biol Chem. 1979;254:11495–11504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

