Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2
- PMID: 19595719
- PMCID: PMC2744448
- DOI: 10.1016/j.molcel.2009.05.012
Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2
Abstract
Substrates of the E3 ubiquitin ligase CRL4(Cdt2), including Cdt1 and p21, contain a PCNA-binding motif called a PIP box. Upon binding of the PIP box to PCNA on chromatin, CRL4(Cdt2) is recruited and the substrate is ubiquitylated. Importantly, a PIP box cannot be sufficient for destruction, as most PIP box proteins are stable. Using Xenopus egg extracts, we identify two sequence elements in CRL4(Cdt2) substrates that promote their proteolysis: a specialized PIP box that confers exceptionally efficient PCNA binding and a basic amino acid 4 residues downstream of the PIP box, which recruits CRL4(Cdt2) to the substrate-PCNA complex. We also identify two mechanisms that couple CRL4(Cdt2)-dependent proteolysis to the chromatin-bound form of PCNA, ensuring that this proteolysis pathway is active only in S phase or after DNA damage. Thus, CRL4(Cdt2) recognizes an unusual degron, which is assembled specifically on chromatin via the binding of a specialized PIP box to PCNA.
Figures



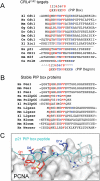
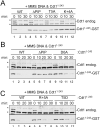


References
-
- Ang XL, Wade Harper J. SCF-mediated protein degradation and cell cycle control. Oncogene. 2005;24:2860–2870. - PubMed
-
- Arias EE, Walter JC. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol. 2006;8:84–90. - PubMed
-
- Chuang LC, Yew PR. Regulation of nuclear transport and degradation of the Xenopus cyclin-dependent kinase inhibitor, p27Xic1. J Biol Chem. 2001;276:1610–1617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

