Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination
- PMID: 19595722
- PMCID: PMC2746756
- DOI: 10.1016/j.molcel.2009.06.019
Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination
Abstract
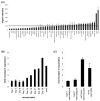
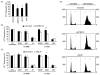
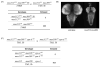
DNA recombination and repair pathways require structure-specific endonucleases to process DNA structures that include forks, flaps, and Holliday junctions. Previously, we determined that the Drosophila MEI-9-ERCC1 endonuclease interacts with the MUS312 protein to produce meiotic crossovers, and that MUS312 has a MEI-9-independent role in interstrand crosslink (ICL) repair. The importance of MUS312 to pathways crucial for maintaining genomic stability in Drosophila prompted us to search for orthologs in other organisms. Based on sequence, expression pattern, conserved protein-protein interactions, and ICL repair function, we determined that the mammalian ortholog of MUS312 is BTBD12. Orthology between these proteins and S. cerevisiae Slx4 helped identify a conserved interaction with a second structure-specific endonuclease, SLX1. Genetic and biochemical evidence described here and in related papers suggest that MUS312 and BTBD12 direct Holliday junction resolution by at least two distinct endonucleases in different recombination and repair contexts.
Figures

References
-
- Aravind L, Koonin EV. SAP - a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. 2000;25:112–114. - PubMed
-
- Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994;265:2082–2085. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

