A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract
- PMID: 19595763
- PMCID: PMC3816373
- DOI: 10.1016/j.bbadis.2009.06.011
A novel mutation (F71L) in alphaA-crystallin with defective chaperone-like function associated with age-related cataract
Abstract
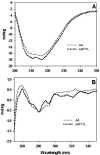
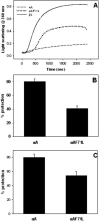
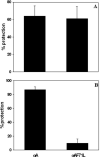
Age-related cataract (ARC) is a multifactorial disease and the leading cause of blindness worldwide. Genetic predisposition in association with other etiological factors may contribute to ARC. However, gene mutation studies on ARC are scanty. In the present work, we identified a genetic variation (F71L) in the exon-2 of CRYAA (alphaA-crystallin) gene in three unrelated female sporadic cases among 711 ARC patients but not in 265 normal non-cataractous controls by SSCP and RFLP analysis. By comparing human recombinant wild-type and F71L-alphaA-crystallin, we characterized the functional significance of this missense mutation. Chromatography, fluorescence and far- and near-UV CD studies indicated that F71L missense mutation did not significantly affect the apparent molecular mass, secondary and tertiary structures and hydrophobicity of alphaA-crystallin. While the mutant alphaA-crystallin displayed significant (35-90%) loss of chaperone-like activity (CLA) in thermal aggregation of carbonic anhydrase, betaL- and gamma-crystallins, it showed moderate (10-50%) loss in CLA in DTT-induced aggregation of insulin and lysozyme. This is the first report of an alphaA-F71L mutation being associated with ARC and suggests that ARC in individuals carrying this mutation (F71L) might be due to the overall loss of in vivo chaperone activity due to interaction with other environmental factors.
Figures



References
-
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. - PubMed
-
- McCarty, Taylor H. The genetics of cataract. Invest Ophthalmol Vis Sci. 2001;42:1677–8. - PubMed
-
- Leske MC, Chylack LT, Jr, Wu SY. The Lens Opacities Case-Control Study. Risk factors for cataract. Arch. Ophthalmol. 1991;109:244–51. - PubMed
-
- The Italian-American Cataract Study Group Incidence and progression of cortical, nuclear, and posterior subcapsular cataracts. Am J Ophthalmol. 1994;118:623–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

