The toll-like receptor 3:dsRNA signaling complex
- PMID: 19595807
- PMCID: PMC2784288
- DOI: 10.1016/j.bbagrm.2009.06.005
The toll-like receptor 3:dsRNA signaling complex
Abstract
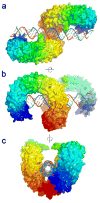
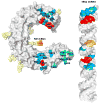
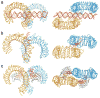
Toll-like receptors (TLRs) recognize conserved molecular patterns in invading pathogens and trigger innate immune responses. TLR3 recognizes dsRNA, a molecular signature of most viruses via its ectodomain (ECD). The TLR3-ECD structure consists of a 23 turn coil bent into the shape of a horseshoe with specialized domains capping the N and C-terminal ends of the coil. TLR3-ECDs bind as dimeric units to dsRNA oligonucleotides of at least 45 bp in length, the minimal length required for signal transduction. X-ray analysis has shown that each TLR3-ECD of a dimer binds dsRNA at two sites located at opposite ends of the TLR3 "horseshoe" on the one lateral face that lacks N-linked glycans. Intermolecular contacts between the C-terminal domains of two TLR3-ECDs stabilize the dimer and position the C-terminal residues within 20-25 A of each other, which is thought to be essential for transducing a signal across the plasma membrane in intact TLR3 molecules. Interestingly, in TLRs 1, 2 and 4, which bind lipid ligands using very different interactions from TLR3, the ligands nevertheless promote the formation of a dimer in which the same two lateral surfaces as in the TLR3-ECD:dsRNA complex face each other, bringing their C-termini in close proximity. Thus, a pattern is emerging in which pathogen-derived substances bind to TLR-ECDs, thereby promoting the formation of a dimer in which the glycan-free ligand binding surfaces face each other and the two C-termini are brought in close proximity for signal transduction.
Figures



References
-
- Bell JK, Mullen GE, Leifer CA, Mazzoni A, Davies DR, Segal DM. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 2003;24:528. - PubMed
-
- Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581. - PubMed
-
- de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem. 2005;280:38133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

