Identification of the neural pathway underlying spontaneous crossed phrenic activity in neonatal rats
- PMID: 19596054
- PMCID: PMC2760647
- DOI: 10.1016/j.neuroscience.2009.07.011
Identification of the neural pathway underlying spontaneous crossed phrenic activity in neonatal rats
Abstract
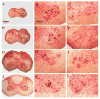
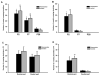
Cervical spinal cord hemisection at C2 leads to paralysis of the ipsilateral hemidiaphragm in rats. Respiratory function of the paralyzed hemidiaphragm can be restored by activating a latent respiratory motor pathway in adult rats. This pathway is called the crossed phrenic pathway and the restored activity in the paralyzed hemidiaphragm is referred to as crossed phrenic activity. The latent neural pathway is not latent in neonatal rats as shown by the spontaneous expression of crossed phrenic activity. However, the anatomy of the pathway in neonatal rats is still unknown. In the present study, we hypothesized that the crossed phrenic pathway may be different anatomically in neonatal and adult rats. To delineate this neural pathway in neonates, we injected wheat germ agglutinin conjugated to horseradish peroxidase (WGA-HRP), a retrograde transsynaptic tracer, into the phrenic nerve ipsilateral to hemisection. We also injected cholera toxin subunit B-horseradish peroxidase (BHRP) into the ipsilateral hemidiaphragm following hemisection in other animals to determine if there are midline-crossing phrenic dendrites involved in the crossed phrenic pathway in neonatal rats. The WGA-HRP labeling was observed only in the ipsilateral phrenic nucleus and ipsilateral rostral ventral respiratory group (rVRG) in the postnatal day (P) 2, P7, and P28 hemisected rats. Bilateral labeling of rVRG neurons was shown in P35 rats. The BHRP study showed that many phrenic dendrites cross the midline in P2 neonatal rats at both rostral and caudal parts of the phrenic nucleus. There was a marked reduction of crossing dendrites observed in P7 and P28 animals and no crossing dendrites observed in P35 rats. The present results suggest that the crossed phrenic pathway in neonatal rats involves the parent axons from ipsilateral rVRG premotor neurons that cross at the level of obex as well as decussating axon collaterals that cross over the spinal cord midline to innervate ipsilateral phrenic motoneurons following C2 hemisection. In addition, midline-crossing dendrites of the ipsilateral phrenic motoneurons may also contribute to the crossed phrenic pathway in neonates.
Figures





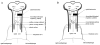
Similar articles
-
Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat.Exp Neurol. 1992 Jun;116(3):219-28. doi: 10.1016/0014-4886(92)90001-7. Exp Neurol. 1992. PMID: 1375167
-
Postnatal conversion of cross phrenic activity from an active to latent state.Exp Neurol. 2009 Sep;219(1):66-73. doi: 10.1016/j.expneurol.2009.01.024. Epub 2009 Feb 10. Exp Neurol. 2009. PMID: 19416665 Free PMC article.
-
The pattern and extent of retrograde transsynaptic transport of WGA-Alexa 488 in the phrenic motor system is dependent upon the site of application.J Neurosci Methods. 2014 Jan 30;222:156-64. doi: 10.1016/j.jneumeth.2013.11.003. Epub 2013 Nov 12. J Neurosci Methods. 2014. PMID: 24239778 Free PMC article.
-
The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury.J Appl Physiol (1985). 2003 Feb;94(2):795-810. doi: 10.1152/japplphysiol.00847.2002. J Appl Physiol (1985). 2003. PMID: 12531916 Review.
-
Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):115-22. doi: 10.1016/j.resp.2009.08.004. Epub 2009 Aug 12. Respir Physiol Neurobiol. 2009. PMID: 19682608 Review.
Cited by
-
Structural and functional identification of two distinct inspiratory neuronal populations at the level of the phrenic nucleus in the rat cervical spinal cord.Brain Struct Funct. 2019 Jan;224(1):57-72. doi: 10.1007/s00429-018-1757-3. Epub 2018 Sep 24. Brain Struct Funct. 2019. PMID: 30251026 Free PMC article.
-
Segmental organization of vestibulospinal inputs to spinal interneurons mediating crossed activation of thoracolumbar motoneurons in the neonatal mouse.J Neurosci. 2015 May 27;35(21):8158-69. doi: 10.1523/JNEUROSCI.5188-14.2015. J Neurosci. 2015. PMID: 26019332 Free PMC article.
-
The crossed phrenic phenomenon.Neural Regen Res. 2017 Jun;12(6):845-864. doi: 10.4103/1673-5374.208539. Neural Regen Res. 2017. PMID: 28761411 Free PMC article. Review.
-
Hypoxia triggers short term potentiation of phrenic motoneuron discharge after chronic cervical spinal cord injury.Exp Neurol. 2015 Jan;263:314-24. doi: 10.1016/j.expneurol.2014.10.002. Epub 2014 Oct 16. Exp Neurol. 2015. PMID: 25448009 Free PMC article.
-
Mid-cervical spinal cord contusion causes robust deficits in respiratory parameters and pattern variability.Exp Neurol. 2018 Aug;306:122-131. doi: 10.1016/j.expneurol.2018.04.005. Epub 2018 Apr 10. Exp Neurol. 2018. PMID: 29653187 Free PMC article.
References
-
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol. 1997;382:469–479. - PubMed
-
- Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res. 2007;1148:96–104. - PubMed
-
- Cameron WE, Nunez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons during postnatal development. Brain Res Bull. 2000;53:523–527. - PubMed
-
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. - PubMed
-
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol. 1990 Dec 22;302(4):707–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

