Duration of coherence intervals in electrical brain activity in perceptual organization
- PMID: 19596712
- PMCID: PMC2803735
- DOI: 10.1093/cercor/bhp107
Duration of coherence intervals in electrical brain activity in perceptual organization
Abstract
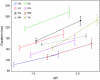
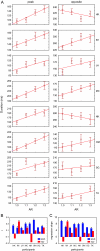
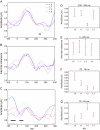
We investigated the relationship between visual experience and temporal intervals of synchronized brain activity. Using high-density scalp electroencephalography, we examined how synchronized activity depends on visual stimulus information and on individual observer sensitivity. In a perceptual grouping task, we varied the ambiguity of visual stimuli and estimated observer sensitivity to this variation. We found that durations of synchronized activity in the beta frequency band were associated with both stimulus ambiguity and sensitivity: the lower the stimulus ambiguity and the higher individual observer sensitivity the longer were the episodes of synchronized activity. Durations of synchronized activity intervals followed an extreme value distribution, indicating that they were limited by the slowest mechanism among the multiple neural mechanisms engaged in the perceptual task. Because the degree of stimulus ambiguity is (inversely) related to the amount of stimulus information, the durations of synchronous episodes reflect the amount of stimulus information processed in the task. We therefore interpreted our results as evidence that the alternating episodes of desynchronized and synchronized electrical brain activity reflect, respectively, the processing of information within local regions and the transfer of information across regions.
Figures










References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Barrie JM, Freeman WJ, Lenhart MD. Spatiotemporal analysis of prepyriform, visual, auditory, and somesthetic surface EEGs in trained rabbits. J Neurophysiol. 1996;76:520–539. - PubMed
-
- Barry RJ, Kirkaikul S, Hodder D. EEG alpha activity and the ERP to target stimuli in an auditory oddball paradigm. Int J Psychophysiol. 2000;39:39–50. - PubMed
-
- Basar E. Dynamics of potentials from invertebrate brain. In: Basar E, editor. Brain functions and oscillations II: Integrative brain function. Neurophysiology and cognitive processes. Berlin: Springer; 1999. pp. 91–108.
-
- Brandt ME, Jansen BH, Carbonari JP. Pre-stimulus spectral EEG patterns and the visual evoked response. Electroencephalogr Clin Neurophysiol. 1991;80:16–20. - PubMed

