The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition
- PMID: 19596789
- PMCID: PMC2738297
- DOI: 10.1128/MCB.00144-09
The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition
Abstract
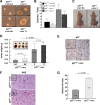
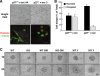
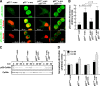
In many human cancers, p27 downregulation correlates with a worse prognosis, suggesting that p27 levels could represent an important determinant in cell transformation and cancer development. Using a mouse model system based on v-src-induced transformation, we show here that p27 absence is always linked to a more aggressive phenotype. When cultured in three-dimensional contexts, v-src-transformed p27-null fibroblasts undergo a morphological switch from an elongated to a rounded cell shape, accompanied by amoeboid-like morphology and motility. Importantly, the acquisition of the amoeboid motility is associated with a greater ability to move and colonize distant sites in vivo. The reintroduction of different p27 mutants in v-src-transformed p27-null cells demonstrates that the control of cell proliferation and motility represents two distinct functions of p27, both necessary for it to fully act as a tumor suppressor. Thus, we highlight here a new p27 function in driving cell plasticity that is associated with its C-terminal portion and does not depend on the control of cyclin-dependent kinase activity.
Figures







References
-
- Baldassarre, G., B. Belletti, M. S. Nicoloso, M. Schiappacassi, A. Vecchione, P. Spessotto, A. Morrione, V. Canzonieri, and A. Colombatti. 2005. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 751-63. - PubMed
-
- Baldassarre, G., B. Belletti, P. Bruni, A. Boccia, F. Trapasso, F. Pentimalli, M. V. Barone, G. Chiappetta, M. T. Vento, S. Spiezia, A. Fusco, and G. Viglietto. 1999. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J. Clin. Investig. 104865-874. - PMC - PubMed
-
- Belletti, B., M. S. Nicoloso, M. Schiappacassi, E. Chimienti, S. Berton, F. Lovat, A. Colombatti, and G. Baldassarre. 2005. p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr. Med. Chem. 121589-1605. - PubMed
-
- Belletti, B., M. S. Nicoloso, M. Schiappacassi, S. Berton, F. Lovat, K. Wolf, V. Canzonieri, S. D'Andrea, A. Zucchetto, P. Friedl, A. Colombatti, and G. Baldassarre. 2008. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol. Biol. Cell 192003-2013. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
