Coupling phosphate homeostasis to cell cycle-specific transcription: mitotic activation of Saccharomyces cerevisiae PHO5 by Mcm1 and Forkhead proteins
- PMID: 19596791
- PMCID: PMC2738290
- DOI: 10.1128/MCB.00222-09
Coupling phosphate homeostasis to cell cycle-specific transcription: mitotic activation of Saccharomyces cerevisiae PHO5 by Mcm1 and Forkhead proteins
Abstract
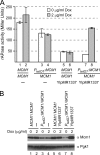
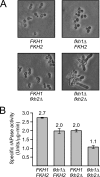
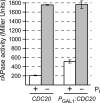
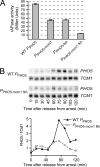
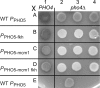
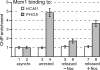
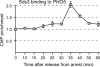
Cells devote considerable resources to nutrient homeostasis, involving nutrient surveillance, acquisition, and storage at physiologically relevant concentrations. Many Saccharomyces cerevisiae transcripts coding for proteins with nutrient uptake functions exhibit peak periodic accumulation during M phase, indicating that an important aspect of nutrient homeostasis involves transcriptional regulation. Inorganic phosphate is a central macronutrient that we have previously shown oscillates inversely with mitotic activation of PHO5. The mechanism of this periodic cell cycle expression remains unknown. To date, only two sequence-specific activators, Pho4 and Pho2, were known to induce PHO5 transcription. We provide here evidence that Mcm1, a MADS-box protein, is essential for PHO5 mitotic activation. In addition, we found that cells simultaneously lacking the forkhead proteins, Fkh1 and Fkh2, exhibited a 2.5-fold decrease in PHO5 expression. The Mcm1-Fkh2 complex, first shown to transactivate genes within the CLB2 cluster that drive G(2)/M progression, also associated directly at the PHO5 promoter in a cell cycle-dependent manner in chromatin immunoprecipitation assays. Sds3, a component specific to the Rpd3L histone deacetylase complex, was also recruited to PHO5 in G(1). These findings provide (i) further mechanistic insight into PHO5 mitotic activation, (ii) demonstrate that Mcm1-Fkh2 can function combinatorially with other activators to yield late M/G(1) induction, and (iii) couple the mitotic cell cycle progression machinery to cellular phosphate homeostasis.
Figures



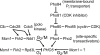
References
-
- Amon, A., M. Tyers, B. Futcher, and K. Nasmyth. 1993. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell 74993-1007. - PubMed
-
- Anderson, M. S., and J. M. Lopes. 1996. Carbon source regulation of PIS1 gene expression in Saccharomyces cerevisiae involves the MCM1 gene and the two-component regulatory gene, SLN1. J. Biol. Chem. 27126596-26601. - PubMed
-
- Barbaric, S., T. Luckenbach, A. Schmid, D. Blaschke, W. Hörz, and P. Korber. 2007. Redundancy of chromatin remodeling pathways for the induction of the yeast PHO5 promoter in vivo. J. Biol. Chem. 28227610-27621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
