Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton
- PMID: 19596800
- PMCID: PMC2909318
- DOI: 10.1242/jcs.043794
Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton
Abstract
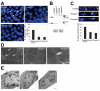
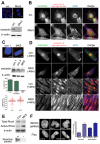
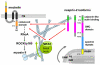
Meckel-Gruber syndrome (MKS) is a severe autosomal recessively inherited disorder caused by mutations in genes that encode components of the primary cilium and basal body. Here we show that two MKS proteins, MKS1 and meckelin, that are required for centrosome migration and ciliogenesis interact with actin-binding isoforms of nesprin-2 (nuclear envelope spectrin repeat protein 2, also known as Syne-2 and NUANCE). Nesprins are important scaffold proteins for maintenance of the actin cytoskeleton, nuclear positioning and nuclear-envelope architecture. However, in ciliated-cell models, meckelin and nesprin-2 isoforms colocalized at filopodia prior to the establishment of cell polarity and ciliogenesis. Loss of nesprin-2 and nesprin-1 shows that both mediate centrosome migration and are then essential for ciliogenesis, but do not otherwise affect apical-basal polarity. Loss of meckelin (by siRNA and in a patient cell-line) caused a dramatic remodelling of the actin cytoskeleton, aberrant localization of nesprin-2 isoforms to actin stress-fibres and activation of RhoA signalling. These findings further highlight the important roles of the nesprins during cellular and developmental processes, particularly in general organelle positioning, and suggest that a mechanistic link between centrosome positioning, cell polarity and the actin cytoskeleton is required for centrosomal migration and is essential for early ciliogenesis.
Figures


References
-
- Adams, M., Smith, U. M., Logan, C. V. and Johnson, C. A. (2008). Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J. Med. Genet. 45, 257-267. - PubMed
-
- Boisvieux-Ulrich, E., Laine, M. and Sandoz, D. (1990). Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 259, 443-454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

