Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration
- PMID: 19596850
- PMCID: PMC2712990
- DOI: 10.1083/jcb.200811071
Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration
Abstract
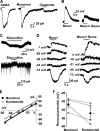
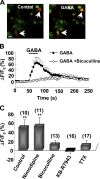
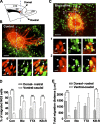
NG2 cells originate from various brain regions and migrate to their destinations during early development. These cells express voltage-gated Na(+) channels but fail to produce typical action potentials. The physiological role of Na(+) channels in these cells is unclear. We found that GABA induces membrane depolarization and Ca(2+) elevation in NG2 cells, a process requiring activation of GABA(A) receptors, Na(+) channels, and Na(+)/Ca(2+) exchangers (NCXs), but not Ca(2+) channels. We have identified a persistent Na(+) current in these cells that may underlie the GABA-induced pathway of prolonged Na(+) elevation, which in turn triggers Ca(2+) influx via NCXs. This unique Ca(2+) signaling pathway is further shown to be involved in the migration of NG2 cells. Thus, GABAergic signaling mediated by sequential activation of GABA(A) receptors, noninactivating Na(+) channels, and NCXs may play an important role in the development and function of NG2 glial cells in the brain.
Figures







References
-
- Aguirre A., Dupree J.L., Mangin J.M., Gallo V. 2007. A functional role for EGFR signaling in myelination and remyelination.Nat. Neurosci. 10:990–1002 - PubMed
-
- Akerman C.J., Cline H.T. 2007. Refining the roles of GABAergic signaling during neural circuit formation.Trends Neurosci. 30:382–389 - PubMed
-
- Annunziato L., Pignataro G., Di Renzo G.F. 2004. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives.Pharmacol. Rev. 56:633–654 - PubMed
-
- Barres B.A., Koroshetz W.J., Swartz K.J., Chun L.L., Corey D.P. 1990. Ion channel expression by white matter glia: the O-2A glial progenitor cell.Neuron. 4:507–524 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

