Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo
- PMID: 19596856
- PMCID: PMC2758008
- DOI: 10.1074/jbc.M109.018473
Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo
Abstract
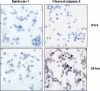
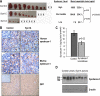
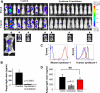
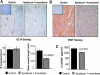
Myeloma tumors are characterized by high expression of syndecan-1 (CD138), a heparan sulfate proteoglycan present on the myeloma cell surface and shed into the tumor microenvironment. High levels of shed syndecan-1 in the serum of patients are an indicator of poor prognosis, and numerous studies have implicated syndecan-1 in promoting the growth and progression of this cancer. In the present study we directly addressed the role of syndecan-1 in myeloma by stable knockdown of its expression using RNA interference. Knockdown cells that were negative for syndecan-1 expression became apoptotic and failed to grow in vitro. Knockdown cells expressing syndecan-1 at approximately 28% or approximately 14% of normal levels survived and grew well in vitro but formed fewer and much smaller subcutaneous tumors in mice compared with tumors formed by cells expressing normal levels of syndecan-1. When injected intravenously into mice (experimental metastasis model), knockdown cells formed very few metastases as compared with controls. This indicates that syndecan-1 may be required for the establishment of multi-focal metastasis, a hallmark of this cancer. One mechanism of syndecan-1 action occurs via stimulation of tumor angiogenesis because tumors formed by knockdown cells exhibited diminished levels of vascular endothelial growth factor and impaired development of blood vessels. Together, these data indicate that the effects of syndecan-1 on myeloma survival, growth, and dissemination are due, at least in part, to its positive regulation of tumor-host interactions that generate an environment capable of sustaining robust tumor growth.
Figures



References
-
- Barlogie B., Shaughnessy J., Epstein J., Sanderson R., Anaissie E., Walker R., Tricot G. (2006) in Williams Hematology ( Lichtman M. A., Beutler E., Kipps T. J., Seligsohn U., Kaushansky K., Prchal J. T. eds) 7th Ed., pp. 1501–1533, McGraw-Hill, New York
-
- Anderson K. C. (2007) Exp. Hematol. 35, 155–162 - PubMed
-
- Roodman G. D. (2002) J. Bone Miner. Res. 17, 1921–1925 - PubMed
-
- Caers J., Van Valckenborgh E., Menu E., Van Camp B., Vanderkerken K. (2008) Bull. Cancer 95, 301–313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

