Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis
- PMID: 19596875
- PMCID: PMC2764163
- DOI: 10.1128/AAC.00341-09
Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis
Abstract
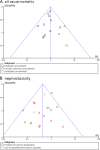
Vancomycin and teicoplanin are the glycopeptides currently in use for the treatment of infections caused by invasive beta-lactam-resistant gram-positive organisms. We conducted a systematic review and meta-analysis of randomized controlled trials that have compared vancomycin and teicoplanin administered systemically for the treatment of suspected or proven infections. A comprehensive search of trials without year, language, or publication status restrictions was performed. The primary outcome was all-cause mortality. Two reviewers independently extracted the data. Risk ratios (RRs) with 95% confidence intervals (CIs) were pooled by using the fixed-effect model (RRs of >1 favor vancomycin). Twenty-four trials were included. All-cause mortality was similar overall (RR, 0.95; 95% CI, 0.74 to 1.21), and there was no significant heterogeneity. In trials that used adequate allocation concealment, the results favored teicoplanin (RR, 0.82; 95% CI, 0.63 to 1.06), while in trials with unknown methods or inadequate concealment, the results favored vancomycin (RR, 3.61; 95% CI, 1.27 to 10.30). The latter trials might have recruited more severely ill patients. No other variable affected the RRs for mortality, including the assessment of glycopeptides administered empirically or for proven infections, neutropenia, the participant's age, and drug dosing. There were no significant differences between teicoplanin and vancomycin with regard to clinical failure (RR, 0.92; 95% CI, 0.81 to 1.05), microbiological failure (RR, 1.24; 95% CI, 0.93 to 1.65), and other efficacy outcomes. Lower RRs (in favor of teicoplanin) for clinical failure were observed with a lower risk of bias and when treatment was initiated for infections caused by gram-positive organisms rather than empirically. Total adverse events (RR, 0.61; 95% CI, 0.50 to 0.74), nephrotoxicity (RR, 0.44; 95% CI, 0.32 to 0.61), and red man syndrome were significantly less frequent with teicoplanin. Teicoplanin is not inferior to vancomycin with regard to efficacy and is associated with a lower adverse event rate than vancomycin.
Figures



References
-
- Akan, H. 2008. Comparison of teicoplanin and vancomycin in initial empirical antibiotic regimen for febrile neutropenic patients. Trial NCT00454272. National Institutes of Health, Bethesda, MD. http://clinicaltrials.gov/.
-
- Alfandari, S., C. Bonenfant, L. Depretere, and G. Beaucaire. 2007. Use of 27 parenteral antimicrobial agents in north of France hospitals. Med. Mal. Infect. 37:103-107. - PubMed
-
- Ansari, F., K. Gray, D. Nathwani, G. Phillips, S. Ogston, C. Ramsay, and P. Davey. 2003. Outcomes of an intervention to improve hospital antibiotic prescribing: interrupted time series with segmented regression analysis. J. Antimicrob. Chemother. 52:842-848. - PubMed
-
- Auperin, A., C. Cappelli, E. Benhamou, A. Pinna, E. Peeters, C. Atlani, and O. Hartmann. 1997. Teicoplanin or vancomycin in febrile neutropenic children with cancer: a randomized study on cost effectiveness. Med. Mal. Infect. 27:984-988. (In French.)
-
- Boucher, H. W., and G. R. Corey. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S344-S349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

