Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids
- PMID: 19596877
- PMCID: PMC2764178
- DOI: 10.1128/AAC.00428-09
Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids
Abstract
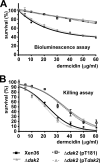
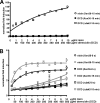
Antimicrobial peptides (AMPs) form an important part of the innate host defense. In contrast to most AMPs, human dermcidin has an anionic net charge. To investigate whether bacteria have developed specific mechanisms of resistance to dermcidin, we screened for mutants of the leading human pathogen, Staphylococcus aureus, with altered resistance to dermcidin. To that end, we constructed a plasmid for use in mariner-based transposon mutagenesis and developed a high-throughput cell viability screening method based on luminescence. In a large screen, we did not find mutants with strongly increased susceptibility to dermcidin, indicating that S. aureus has no specific mechanism of resistance to this AMP. Furthermore, we detected a mutation in a gene of unknown function that resulted in significantly increased resistance to dermcidin. The mutant strain had an altered membrane phospholipid pattern and showed decreased binding of dermcidin to the bacterial surface, indicating that dermcidin interacts with membrane phospholipids. The mode of this interaction was direct, as shown by assays of dermcidin binding to phospholipid preparations, and specific, as the resistance to other AMPs was not affected. Our findings indicate that dermcidin has an exceptional value for the human innate host defense and lend support to the idea that it evolved to evade bacterial resistance mechanisms targeted at the cationic character of most AMPs. Moreover, they suggest that the antimicrobial activity of dermcidin is dependent on the interaction with the bacterial membrane and might thus assist with the determination of the yet unknown mode of action of this important human AMP.
Figures




References
-
- Bae, T., and O. Schneewind. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58-63. - PubMed
-
- Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. - PubMed
-
- Bruckner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

