North American paragonimiasis (Caused by Paragonimus kellicotti) in the context of global paragonimiasis
- PMID: 19597007
- PMCID: PMC2708389
- DOI: 10.1128/CMR.00005-08
North American paragonimiasis (Caused by Paragonimus kellicotti) in the context of global paragonimiasis
Abstract
Paragonimus species are highly evolved parasites with a complex life cycle that involves at least three different hosts, i.e., snails, crustaceans, and mammals. The adult forms of Paragonimus species reside and mate in the lungs of a variety of permissive mammalian hosts, including humans. Although human paragonimiasis is uncommonly encountered in North America, both autochthonous and imported disease may be encountered. Paragonimus kellicotti, the species endemic to North America, is a well-known pathogen in wild and domestic animals. Five patients with North American paragonimiasis have been reported in the recent medical literature. The biologic, clinical, radiologic, and laboratory features of paragonimiasis are reviewed, with emphasis on North American paragonimiasis whenever possible.
Figures

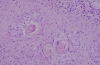



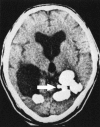



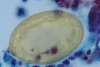



Similar articles
-
Serological diagnosis of North American Paragonimiasis by Western blot using Paragonimus kellicotti adult worm antigen.Am J Trop Med Hyg. 2013 Jun;88(6):1035-40. doi: 10.4269/ajtmh.12-0720. Epub 2013 Apr 15. Am J Trop Med Hyg. 2013. PMID: 23589531 Free PMC article.
-
North American paragonimiasis. A case report.Acta Cytol. 2000 Jan-Feb;44(1):75-80. doi: 10.1159/000326230. Acta Cytol. 2000. PMID: 10667165
-
Paragonimus kellicotti flukes in Missouri, USA.Emerg Infect Dis. 2012 Aug;18(8):1263-7. doi: 10.3201/eid1808.120335. Emerg Infect Dis. 2012. PMID: 22840191 Free PMC article.
-
Human paragonimiasis in North America following ingestion of raw crayfish.Clin Infect Dis. 2009 Sep 15;49(6):e55-61. doi: 10.1086/605534. Clin Infect Dis. 2009. PMID: 19681705 Review.
-
A clinical review of human disease due to Paragonimus kellicotti in North America.Parasitology. 2022 Sep;149(10):1327-1333. doi: 10.1017/S0031182021001359. Epub 2021 Jul 27. Parasitology. 2022. PMID: 35965058 Free PMC article. Review.
Cited by
-
New Nodule Type Found in the Lungs of Pomacea canaliculata, an Intermediate Host of Angiostrongylus cantonensis.Iran J Parasitol. 2018 Jul-Sep;13(3):362-368. Iran J Parasitol. 2018. PMID: 30483326 Free PMC article.
-
Molecular characterization of the North American lung fluke Paragonimus kellicotti in Missouri and its development in Mongolian gerbils.Am J Trop Med Hyg. 2011 Jun;84(6):1005-11. doi: 10.4269/ajtmh.2011.11-0027. Am J Trop Med Hyg. 2011. PMID: 21633042 Free PMC article.
-
Diagnosis of Human Trematode Infections.Adv Exp Med Biol. 2024;1454:541-582. doi: 10.1007/978-3-031-60121-7_14. Adv Exp Med Biol. 2024. PMID: 39008275 Review.
-
Granulomatous Diseases of the Central Nervous System.Curr Neurol Neurosci Rep. 2022 Jan;22(1):33-45. doi: 10.1007/s11910-022-01173-y. Epub 2022 Feb 9. Curr Neurol Neurosci Rep. 2022. PMID: 35138588 Review.
-
Paragonimiasis.Adv Exp Med Biol. 2024;1454:203-238. doi: 10.1007/978-3-031-60121-7_6. Adv Exp Med Biol. 2024. PMID: 39008267 Review.
References
-
- Abend, L. 1910. Ueber Haemoptysis Parasitaria. Dtsch. Arch. Klin. Med. 100:501-511.
-
- Ameel, D. 1934. Paragonimus, its life history and distribution in North America and its taxonomy. Am. J. Hyg. 19:270-317.
-
- Reference deleted.
-
- Ameel, D. J., W. W. Cort, and A. van der Woude. 1951. Development of the mother sporocyst and rediae of Paragonimus kellicotti Ward, 1908. J. Parasitol. 37:395-404. - PubMed
-
- Amunarriz, M. 1991. Intermediate hosts of Paragonimus in the eastern Amazonic region of Ecuador. Trop. Med. Parasitol. 42:164-166. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

