Resistance to therapies for infection by Plasmodium vivax
- PMID: 19597012
- PMCID: PMC2708388
- DOI: 10.1128/CMR.00008-09
Resistance to therapies for infection by Plasmodium vivax
Abstract
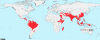
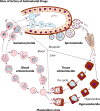
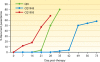
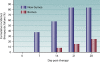
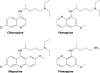
The gravity of the threat posed by vivax malaria to public health has been poorly appreciated. The widely held misperception of Plasmodium vivax as being relatively infrequent, benign, and easily treated explains its nearly complete neglect across the range of biological and clinical research. Recent evidence suggests a far higher and more-severe disease burden imposed by increasingly drug-resistant parasites. The two frontline therapies against vivax malaria, chloroquine and primaquine, may be failing. Despite 60 years of nearly continuous use of these drugs, their respective mechanisms of activity, resistance, and toxicity remain unknown. Although standardized means of assessing therapeutic efficacy against blood and liver stages have not been developed, this review examines the provisional in vivo, ex vivo, and animal model systems for doing so. The rationale, design, and interpretation of clinical trials of therapies for vivax malaria are discussed in the context of the nuance and ambiguity imposed by the hypnozoite. Fielding new drug therapies against real-world vivax malaria may require a reworking of the strategic framework of drug development, namely, the conception, testing, and evaluation of sets of drugs designed for the cure of both blood and liver asexual stages as well as the sexual blood stages within a single therapeutic regimen.
Figures
References
-
- Akintowa, A., M. C. Meyer, and P. T. R. Hwang. 1983. Simultaneous determination of chloroquine and desethylchloroquine in blood, plasma, and urine by high-performance liquid chromatography. J. Liq. Chromatogr. 6:1513-1522.
-
- Alvarez, G., J.-G. Pineros, A. Tobon, A. Rios, A. Maestre, S. Blair, and J. C. Fonseca. 2006. Efficacy of three chloroquine-primaquine regimens for treatment of Plasmodium vivax malaria in Colombia. Am. J. Trop. Med. Hyg. 75:605-609. - PubMed
-
- Alving, A. S., D. D. Hankey, G. R. Coatney, R. Jones, Jr., W. G. Coker, P. L. Garrison, and W. N. Donovan. 1953. Korean vivax malaria. II. Curative treatment with pamaquine and primaquine. Am. J. Trop. Med. Hyg. 2:970-976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

