Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges
- PMID: 19597023
- PMCID: PMC2734400
- DOI: 10.1200/JCO.2009.22.3701
Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges
Abstract
Purpose: Biomarkers can add substantial value to current medical practice by providing an integrated approach to prediction using the genetic makeup of the tumor and the genotype of the patient to guide patient-specific treatment selection. We discuss and evaluate various clinical trial designs for the validation of biomarker-guided therapy.
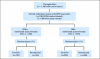
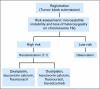
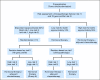
Methods: Designs for predictive marker validation are broadly classified as retrospective (ie, using data from previously well-conducted randomized controlled trials [RCTs]) versus prospective (enrichment, unselected, hybrid, or adaptive analysis). We discuss the salient features of each design in the context of real trials.
Results: Well-designed retrospective analysis from well-conducted prospective RCTs can bring forward effective treatments to marker-defined subgroups of patients in a timely manner (eg, KRAS and colorectal cancer). Enrichment designs are appropriate when preliminary evidence suggest that patients with or without that marker profile do not benefit from the treatments in question; however, this may sometimes leave questions unanswered (eg, trastuzumab and breast cancer). An unselected design is optimal where preliminary evidence regarding treatment benefit and assay reproducibility is uncertain (eg, epidermal growth factor receptor and lung cancer). Hybrid designs are appropriate when preliminary evidence demonstrate the efficacy of certain treatments for a marker-defined subgroup, making it unethical to randomly assign patients with that marker status to other treatments (eg, multigene assay and breast cancer). Adaptive analysis designs allow for prespecified marker-defined subgroup analyses of data from an RCT.
Conclusion: The implementation of these design strategies will lead to a more rapid clinical validation of biomarker-guided therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Taube SE, Jacobson JW, Lively TG. Cancer diagnostics: Decision criteria for marker utilization in the clinic. Am J Pharmacogenomics. 2005;5:357–364. - PubMed
-
- Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non–small-cell lung cancer. J Clin Oncol. 2007;25:587–595. - PubMed
-
- Bonomi PD, Buckingham L, Coon J. Selecting patients for treatment with epidermal growth factor tyrosine kinase inhibitors. Clin Cancer Res. 2007;13(suppl 2):s4606–s4612. - PubMed
-
- Slamon D. Herceptin: Increasing survival in metastatic breast cancer. Eur J Oncol Nurs. 2000;4:24–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

